XENOTRANSPLANTATION: The urgency to prioritize more ethical alternatives
Prioritising more ethical alternatives to xenotransplantation
Thanks to advances in medicine and biotechnology, organ transplantation now plays an essential role in saving and improving the quality of many human lives. The biomedicine agency estimates that around fifteen transplants will be carried out every day in France in 2023 [1]. However, it is estimated that around 19 people die every day in Europe while waiting for an organ [2]. As the need continues to increase, the global shortage of human organs represents a real challenge for biomedical research and public health. A possible way to overcome the shortage problem is the transplantation of organs from animals, what is called “xenotransplantation, XTP, or even xenograft or xenotransplant”.
In this article, we will analyse how and why science became interested in this practice. We will discuss the issues and limits of XTP, and we will explore the reasons why it is urgent to focus on new scientifically promising, more ethical and sustainable approaches.
A scientifically and ethically questionable approach
The idea of transplanting animal organs into humans is a very old one. The first attempts at human xenotransplantation took place at the beginning of the 20th century and ended in failure. At the time, doctors knew little about how the immune system worked and did not understand the mechanisms of rejection that contributed to the failure of operations. In 1984, XTP came to public attention in a particularly dramatic way with the case of “Baby Fae”, a 15-day-old human infant who received the heart of a baboon during an operation in California. The baby survived only twenty days after surgery [3]. This episode shook the scientific community and sparked a debate on the health and ethical consequences of such operations.
Although this ‘breakthrough’ had no immediate impact, xenograft technology has nevertheless been the subject of numerous studies ever since. In the 1990s, scientists began to gain a better understanding of how the immune system works and of the molecular mechanisms underlying rejection. We now know that immune cells recognise the molecules on the surface of each individual’s own cells and distinguish between ‘self’, cells that carry the right markers, and ‘non-self’, cells that carry different markers, which prompt our immune system to attack and destroy any foreign cell or organ. This led to the idea of genetically manipulating animals to bypass the human immune system and make their organs more compatible with our bodies. Pigs have become the species of choice over non-human primates (NHPs) because they are easier to access, their organs are anatomically similar to humans, they have a shorter reproductive maturity and gestation period, and they have more offspring, making them more suitable for genetic manipulation. What’s more, its use raises fewer ethical concerns, as it is already used for human food [4].
Scientific advances in immunology and gene editing (with technologies such as cloning or CRISPR/Cas9) have led to greater success with organs from pigs that have been genetically modified to avoid rejection. However, despite these efforts, all XTP with whole organs has failed in humans. In addition to the immunological problems, there are other physiological limitations that rarely seem to be discussed, such as the pig’s lifespan (around 15 years) and body temperature (39 degrees). Thus, the extensive research into ways of overcoming rejection mechanisms could be completely hampered by the rapid ageing of the transplanted organ or the malfunctioning of porcine enzymes at 37 degrees [5]. This would be particularly serious for the elderly, who have the greatest interest in receiving a long-lasting organ. No data are available on tissue degradation and the actual survival time of the pig organ after XTP.
The importance of quality of life compared to a simple extension must also be taken into account in the equation, given that currently all people who have undergone XTP have lived in extremely difficult conditions (heavy immunosuppressive treatments with high risks of infection, cancer, etc.). The human body naturally has mechanisms to prevent the development of foreign bodies (cells, viruses, bacteria) that could harm the way the body functions. From this point of view, isn’t it counter-productive or even dangerous to try at all costs to bypass these natural protection systems? In 2022 and 2024, the tragic end of patients at best two months after their pig heart or kidney xenotransplants can only confirm these statements. Despite the — apparently exaggerated — optimism of the media and the scientific press, the reality is that science is far from being able to achieve this kind of “feat” [6],[7].
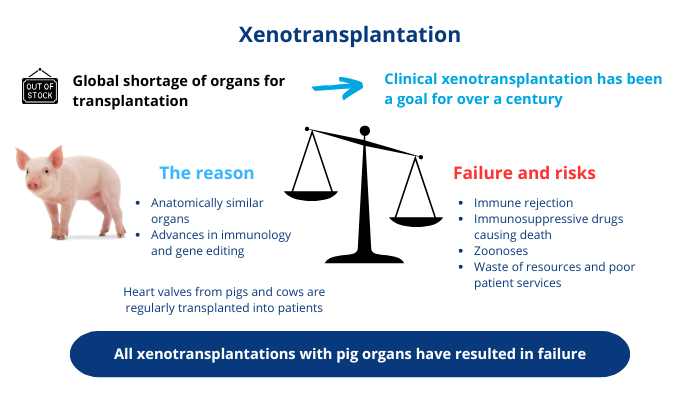
Scheme summarising the current situation of XTP
As well as being dangerous and even lethal for the patient, XTP also entails a real societal risk for global human health by allowing the transmission of inter-species viruses, the source of zoonoses. As recipients are given immunosuppressants to prevent rejection, the risk of infection by animal diseases transmitted by the graft is increased. Once in the patient’s body, the pathogen can not only kill them, but can also mutate and become transmissible to humans, potentially generating pandemics. The degree of risk of propagation is currently unknown because too few clinical trials have been carried out, so it is impossible to rule out the possibility of a new pathogen being transferred from pigs to humans following an XTP [8]. And history shows that these concerns are borne out: a recent MIT Technology Review report mentions the presence of a porcine virus in the body of the first patient to receive a cardiac xenotransplant, who died two months later, most probably as a result of the virus. The biotech company Revivicor, which supplied the genetically modified pig heart, declined to comment [9].
Humankind has already experienced the harmful effects of various zoonotic diseases on several occasions (AIDS, Covid-19, monkey pox, etc.), and it clearly seems ill-advised to open up a whole new route for the transmission of inter-species diseases without further thought. In this sense, xenotransplantation concerns not just doctors, patients and their families, but society as a whole. It is therefore important and necessary to establish a balance between individual benefits and collective costs and risks. The merits of XTP can only be discussed as part of an international debate, because we are talking here about the risk of a pandemic, leading to strict, reasonable and ethical laws and guidelines.
Public opinion and bioethical issues
There are many issues surrounding xenotransplantation. We have already mentioned the health, scientific and ethical issues. These inevitably give rise to a public debate, essentially institutionalised, around the justification and implementation of this approach.
Few objective data currently exist on the public’s reaction to the XTP issue. A study published in Nature at the end of the 90s showed that there were already major differences of opinion between scientists developing and practising XTP, and other members of the medical profession or the general public [10].
Two major social barriers to XTP can be identified:
- Morality or reason: to accept XTP would be to demonstrate speciesism, to accept the right that some humans grant themselves to sacrifice animals in order to benefit from their organs. There is also the question of instrumentalisation, considering animals as tools that can be modified at will [4]. Unlike humans, animals cannot give their consent. What’s more, the manufacture of genetically modified animal organs requires specific facilities and protocols that hinder animal welfare. There is a real bioethical question here concerning respect for human dignity and animal welfare; how far are humans prepared to go in the hope of prolonging the lives of individuals of their species? [11] Is this a reasonable approach, given all the risks (direct and indirect) of human and animal suffering involved?
- Fear: lack of confidence in this unproven approach, psychological discomfort in accepting to carry an organ from an animal of another species (crossing the inter-species barrier). In this sense, over and above fears of suffering physically or even dying as a result of the operation, the testimonies reveal a resistance associated with a fear of losing one’s identity [12]. XTP and genetic modifications carried out on animals also raise the hypothesis that animals acquire human characteristics or the opposite.
In 2023, a survey of more than 5000 people was carried out in the United States. 64% said they were not open to experimental xenotransplantation, even if it would prolong their lives [13]. So it would seem that public opinion has not really changed over the last 30 years, despite the enthusiasm of the press and certain scientists who maintain that XTP will soon be a reality for human health [14], [15].
Even if the scientific barriers and health risks were overcome, society’s acceptance of XTP would remain a major problem, which could mean that this practice never becomes a reality.
Promising, more ethical alternatives
In 1995, organ transplant pioneer Sir Roy Calne declared that xenotransplantation “is just around the corner, but it may be a very long way off” [16]. Thirty years on, no xenotransplant has saved the life of a human being. But what other method could enable the production of functional human organs outside the body — the long-sought ‘Holy Grail’ of regenerative medicine?
In this era of the explosion of ever more complex and innovative (bio)technological tools, several alternative solutions exist, including 3D/4D bioprinting and organoids.
Scientists use a bio-compatible ink (such as collagen), healthy cells from future recipients, and a 3D printer to assemble tissues or organs. According to Dr Luciano Vidal, a doctor and co-founder of the French regenerative medicine biotech company HealShape, we could be transplanting completely bio-printed organs in 8 years’ time [17].
New research carried out by the Wyss Institute and the School of Engineering and Applied Science (SEAS) at Harvard University in the USA has taken a major step towards achieving this goal [18 ]. The researchers have developed a new 3D bio-printing method, coaxial SWIFT (co-SWIFT), which recapitulates the multilayered architecture of native blood vessels [19]. Not only do these biomimetic vessels display the characteristics of human blood vessels, but after five days of perfusion with a fluid that mimics blood, the heart organ building blocks began to beat synchronously, a sign that the heart tissue is healthy and functional.
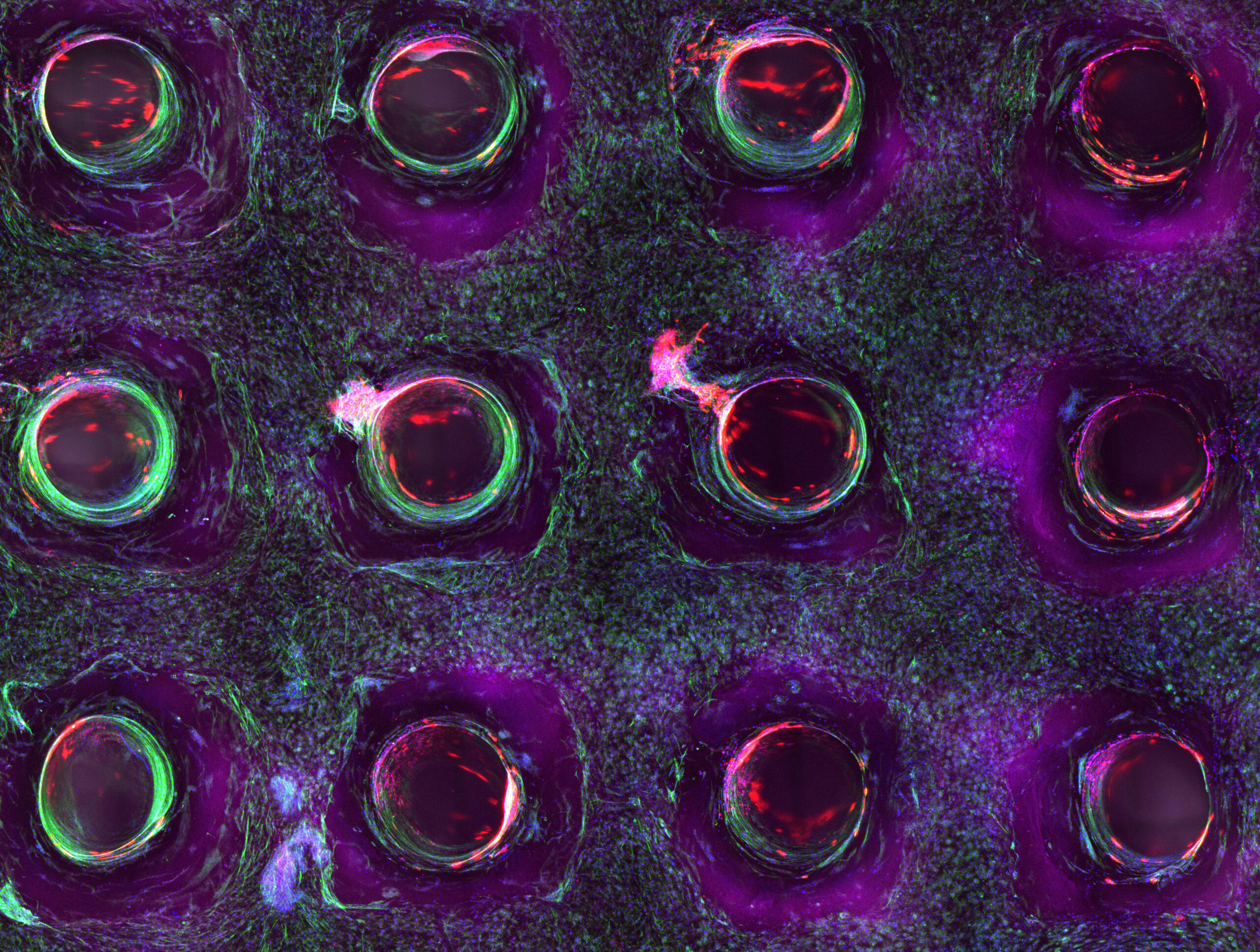
3D bioprinting of living tissue ©Wyss Institute at Harvard University
Organoids, complex 3D structures made up of multiple cell types derived from patients, could also one day serve as an alternative source of organs for transplantation. For example, American researchers at the Center for Stem Cell and Organoid Medicine (CuSTOM) are planning clinical trials of organoid tissue transplants in patients suffering from intestinal problems [20].
3D bioprinting and organoids offer a number of advantages. Perfect biocompatibility, with organs produced from the patient’s own cells. The risk of rejection is therefore much lower, with no need to use cocktails of immunosuppressants, making the procedure less cumbersome and increasing the chances of survival in optimal conditions. These methods pose no risk of pandemic, ethical or bioethical problems, and have definite economic advantages, although these are difficult to assess at present.
Conclusion
With the rise of new biotechnologies that guarantee greater success and lower risks for humans, we may well ask ourselves what is the point of continuing to invest time and money, but also, and above all, to impose physical and psychological suffering on human and animal lives in a process whose scientific and ethical drawbacks are so obvious and whose chances of success so remote. The future of new technologies is such that we could have functional organs on demand, adapted to the patient’s body, within a timeframe that defies all current statistics [21].
Clearly, these new techniques still require a great deal of research and exploration. Transplant experts are impatient for more data to make these new opportunities a reality. As a result, the current huge investment of money and time in XTP research makes less sense and should instead be allocated to these new approaches, which are now possible, less risky and therefore more desirable. The issue of organ shortage is a major one, and it is the responsibility of institutions and the scientific community to devote much more time and resources to these more ethical and sustainable solutions, which are equal to the challenges of our century.
|
Definition and background [22] The term “xenotransplantation” is made up of the Greek root ” xeno ” meaning “foreigner ” and the Latin word “transplantatum” meaning “torn from its place and replanted elsewhere”. “foreign”, and the Latin word ” transplantatum ” meaning “torn from its place and replanted elsewhere”. Originally referring to plants, the term transplant was extended to organs in the context of medicine, meaning “to graft into a living organism an organ taken from another individual”.
|
References
[1] Le rapport médical et scientifique du prélèvement et de la greffe en France (2023).
[2] Journée européenne du don d’organes, de tissus et de cellules.
[3] Bailey LL et al., « Baboon-to-human cardiac xenotransplantation in a neonate » JAMA. 1985 Dec 20;254(23):3321 – 9.
[4] Catherine Rémy, « Le cochon est-il l’avenir de l’homme ? », Terrain, 52 | 2009, 112 – 125.
[5] Dooldeniya MD, Warrens AN. « Xenotransplantation: where are we today? » J R Soc Med. 2003 Mar;96(3):111 – 7.
[6] Quentin Haroche, « Décès du deuxième homme greffé d’un cœur de porc », JIM 2023 7 Novembre.
[7] Smriti Mallapatyn, « First pig liver transplanted into a person lasts for 10 days », Nature 627, 710 – 711 (2024).
[8] Fishman Jay A. et al., « Xenotransplantation-associated infectious risk: a WHO consultation », Xenotransplantation. 2012 Mar-Apr;19(2):72 – 81.
[9] Antonio Regalado, « The gene-edited pig heart given to a dying patient was infected with a pig virus », MIT Technology Review 2022 4 Mai.
[10] Mohacsi, et al., « Aversion to xenotransplantation », Nature 378, 434 (1995).
[11] SHAW D. et al., « Ethical issues surrounding the transplantation of organs from animals into humans », Scientific & Technical Review. 2018 04 1; 37(1): pp. 123 – 129.
[12] Julie Lesage, « Les enjeux éthiques et juridiques de la xénotransplantation », Village de la Justice (2022) 14 Fév.
[13] Padilla Luz A, et al., « Public attitudes to xenotransplantation: A national survey in the United States », American Journal of Transplantation (2024) July 23.
[14] « Xénotransplantation : pour la 1re fois, la réponse immunitaire après la greffe de reins de porcs génétiquement modifiés chez l’humain est décryptée », Communiqué de Presse INSERM, (2023) 18 Août.
[15] Vadori M, Cozzi E., « Current challenges in xenotransplantation », Curr Opin Organ Transplant. 2024 Jun 1;29(3):205 – 211.
[16] Cooper DK., « A brief history of cross-species organ transplantation », Proc (Bayl Univ Med Cent). 2012 Jan;25(1):49 – 57.
[17] Luciano Vidal, « La bio-impression 3D, une révolution pour la médecine régénérative», TEDxRennes, YouTube (2023).
[18] Stankey, Paul P et al. « Embedding Biomimetic Vascular Networks via Coaxial Sacrificial Writing into Functional Tissue », Advanced materials (Deerfield Beach, Fla.) vol. 36,36 (2024): e2401528.
[19] Lindsay Brownell, « 3D-printed blood vessels bring artificial organs closer to reality », Wyss Institute at Harvard University, (2024) August 7.
[20] Science/AAAS Custom Publishing Office, « Organoids: Today’s research tool, tomorrow’s organ transplant solution », Science (2024) March 15.
[21] Brian Kateman, « Pigs aren’t the future of organ tranplants — stop acting like they could be », Fast Company (2024) May 20.
[22] Cooper DKC, et al., « A brief history of clinical xenotransplantation. Int J Surg », 2015 Nov;23(Pt B):205 – 210.
Written by Dr Lilas Courtot, Scientific Manager — Pro Anima Scientific Committee
With the contribution of Julia Fernandez, Scientific mediator — University of Bordeaux
Image credits: Futuro Prossimo (banner), Comité Pro Anima (image 1), Wyss Institute — Harvard University (image 2)


