Advancing biomedical research
The crucial role of Organ-on-Chip technology and strategies for widespread adoption
1 July 2024The crucial role of Organ-on-Chip technology and strategies for widespread adoption
Guest article by Dr. Juliane Fischer, Dynamic42

Organ-on-Chip (OoC) technology represents a revolutionary leap in the field of biomedical and preclinical research, offering unprecedented opportunities to replicate the complex physiology of human organs. As the demand for more accurate and efficient models for drug testing, disease modelling, and personalized medicine grows, the importance of widespread adoption of OoC technology cannot be overstated. This article explores the general significance of OoC, the critical need for technology adoption, and outlines key strategies and prerequisites to achieve this.
As introduced by Prof Don Ingber in the previous edition of this magazine, OoC are microphysiological systems that mimic the human organ tissue in vitro. Traditional in vitro and animal models often fall short in accurately representing human organ function and composition. OoC technology bridges this gap by providing human relevant data and potentially reducing translational failure in drug development.
The potential of Organ-on-chip technology
The technology holds potential for several research fields such as precision or personalized medicine, drug development and biomedical research. In the following chapter, we will explore the significance for each of them, delving into specific use cases and potential directions.
Personalized medicine
In precision medicine, organ models can enable the development of personalized treatments. It allows to study how an individual’s organ or tumor tissue would respond to a specific drug or therapy holding the potential to tailor treatments to the patients themselves. In particular, immunocompetent Organ-on-chip models have the ability to contribute to personalized treatments by allowing researchers to assess how an individual’s immune system responds to specific drugs or treatments. Sharifi 2019 et. al. developed a foreign body response biochip that allows for integration of human donor derived monocytes (white blood cells) to model the immune response to implant material with the aim to identify a suitable material for the patient. Monocytes, macrophages, and neutrophils also play an important role in tumor development (Binnewies 2018 et. al.). It is therefore critical to develop microphysiological models of tumors that replicate the human biology of a patient including the immune component and thereby allowing for the development of personalized treatments. For this purpose, Dynamic42 is currently establishing a patient-derived pancreatic cancer spheroid-on-a-chip model for screening novel therapeutic approaches.
Drug development
In drug development, typically only 1 out of 5,000 – 10,000 potential drugs from drug discovery will reach approval by the health authorities (Rousseaux 2023 et. al.). The reason for this high dropout rate is in part due to translational failure from preclinical studies conducted in 2D cell culture and animals which are then translated to humans in the clinical phase. The failure rate is estimated to be as high as 92% and the majority is lead back to unexpected toxicity or efficacy of the drug (Marshall 2023 et. al.). The use of complex microphysiological in vitro models has shown to be a potent alternative to animal testing that provides human relevant data for safety and risk assessment. In a recent study conducted by Kaden et. al. they assessed the hepatotoxic effects by the withdrawn antibiotic trovafloxacin in comparison to the non-toxic analogue levofloxacin. Whereas trovafloxacin only induced liver injury in animal models and in preliminary in vitro studies with the addition of inflammatory stimuli such as Lipopolysaccharide (LPS) or under non-human relevant concentrations, the liver-on-chip model developed by Kaden et. al. (Figure 1) was able to predict hepatoxicity at human-relevant concentrations without additional stimuli.
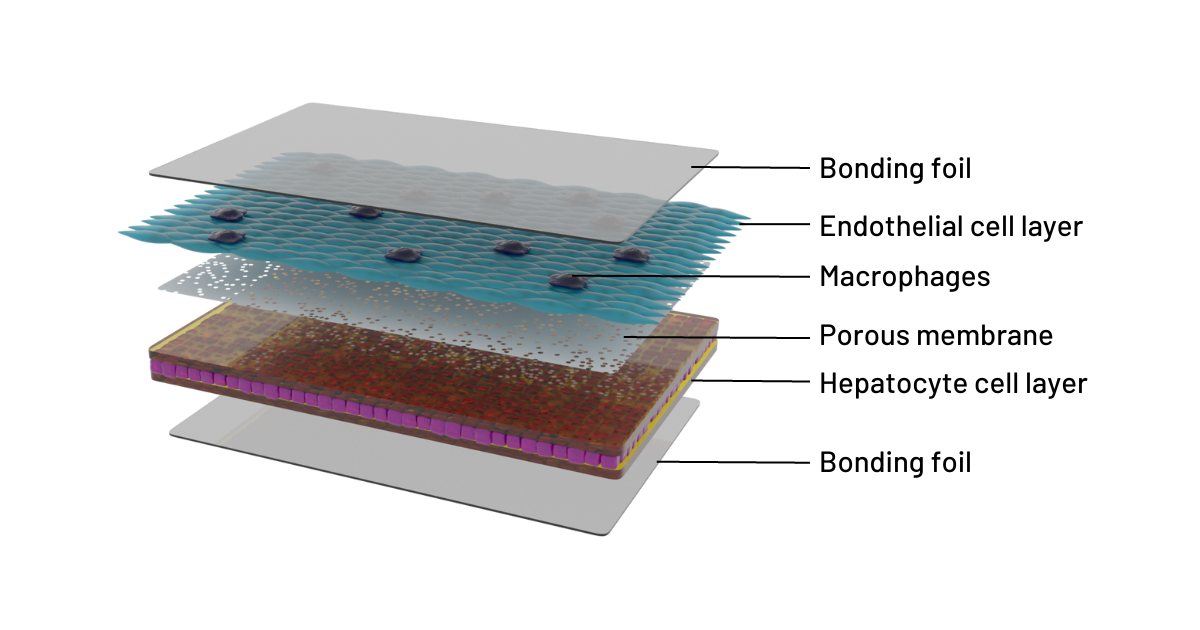
Figure 1 : Cross-sectional illustration of the 3D liver model in the Dynamic42 biochip
In biomedical research
OoC technology holds a large potential for biomedical research. The technology addresses the need for complex disease and infection models replicating the interaction between host and pathogen in a microphysiological environment (Mosig 2016 et.al.). They offer tremendous flexibility and potential for customization specific to the scope and needs of a scientific study including factors that otherwise would only be available in the human body. This can include immune cells, vascularization, the microbiome, pathogens, perfusion and mechanical strain for physiological cell growth as well as multi-organ set ups. In addition to its complexity, the OoC technology allows for a wide range of analysis methods of the studied models during and after the experiment. This can include analysis of barrier function/integrity, compound turnover, oxygen consumption, profiling cytokine release, enzyme activity (transport, metabolization), life-cell imaging, extraction of tissue from the biochip to analyses the models post treatment and many more.
Replacing animal testing
Besides human relevant data, organ models represent a real alternative to animal models as they provide a more accurate representation of the human physiology than classical 2D in vitro models. The reduction and replacement of animal models possess a high motivation for researchers as administrative challenges, time consuming approval processes, high costs and not least, ethical concerns, push for alternative ways to receive physiological data. Although initial setup and development costs may be high for some OoC technologies, the long-term benefits in terms of higher data relevance, streamlining of animal testing, reduced time and cost of drug development, fewer failed clinical trials, increased drug safety and success rates justify the investment.
Evolving regulatory standards
Additionally, the adoption of OoC technology aligns with the increasing emphasis of official authorities on alternative methods to animal testing and provides a platform that meets evolving regulatory standards for drug development and safety assessments (See chapter on changes in legislation below).
How to achieve widespread adoption of OoC technology ?
Education
For any technology to achieve sustainable success it requires widespread adoption by the scientific community, going beyond just the early-adopter phase. Given the complexity of OoC models and their development, education in the method itself will be key to make the technology accessible for industry and academia. It would require its incorporation into biomedical curricula to familiarize future researchers with its principles and applications. And most importantly researchers from industry and academia need access to in-depth, hands-on courses in the technology. Currently, there is a lack of the latter with only 3 in-person OoC technology courses world-wide that offer a regular schedule and hands-on lectures in the lab. In addition, online courses on the technology with method videos and easy to understand explanations of protocols can also enable researchers in remote locations to access information on this technology.
Changes in legislation
Particularly for drug development, changes in legislation and requirements for pre-clinical testing will determine the future success of organ models in this field. Until very recently, regulatory requirements mandated animal toxicity testing of novel drugs prior to their use in human subjects in the clinical phase (Adaschi 2023 et.al.). The FDA Modernization Act 2.0 has now paved the way for the use of alternatives, and non-animal methods are being explored with increasing frequency in drug discovery and development (Science). The European Medicines Agency (EMA) is promoting the principle of the 3Rs by promoting New Approach Methodologies (NAMs) that are in line with the European Union legislation on the protection of animals used for scientific purposes. This is done through EMA’s Innovation Task Force (ITF) which aims to foster dialogue between regulators and developers of medicines to discuss innovative aspects such as emerging therapies, methods and technologies. The ITF is also used for the voluntary submission of data generated with new methods in a so-called safe harbour approach (Candarlioglu 2022 et.al.).
Research funding
The incentive to develop further models to offer alternatives for a variety of animal-based disease and infection models can also make a difference for the field. Especially, research grants focusing on OoC technology can encourage academic institutions and industry partners to invest in this methodology. It also opens funds necessary to establish industry-wide standards for organ models, ensuring consistency and comparability across different models and laboratories.
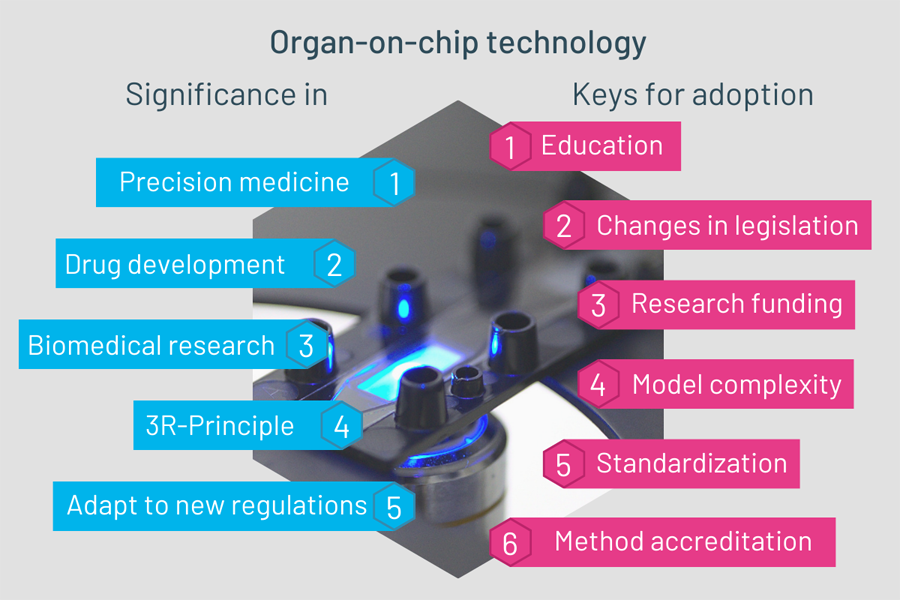
Figure 2 : Significance and keys for adoption of organ-on-chip technology
Model complexity
Finally, the complexity of the models and their ability to really replace animal or other in vitro models will determine their long-term success. To sustain in biomedical research and drug development models need to be able to produce accurate, human relevant data. This requires the models to be as complex and “in-vivo-like” as possible, including features such as :
- Vascularization allowing recapitulation of blood flow
- Integration of multiple cell types at varying complexity
- Perfusion allowing for physiological cell growth
- Integration of microbiome
- Immunocompetence
- Long-term stability
- Adjustable throughput
- Reproducibility
- User-friendliness
Organ-on-chip technology holds immense promise for advancing biomedical research, offering unparalleled opportunities for precision medicine, reducing animal testing, and accelerating drug development. However, achieving widespread adoption requires concerted efforts in education, legislative advocacy, and continuous improvement of OoC models. By incorporating Organ-on-chip into educational curricula, creating specialized courses, advocating for legislative changes, and enhancing the features of OoC models, the biomedical research community can propel this groundbreaking technology into mainstream use, revolutionizing the way we study and understand human physiology and pathology.
About the author

Dr. Juliane Fischer holds a doctoral degree from the Friedrich-Schiller-University in Jena, Germany. Her doctoral work focused on the transcriptional regulation of natural products in filamentous fungi. Transitioning into biotech post-graduation, she specialized in single-cell and microfluidic technologies. Developing an interest in writing and data presentation, she redirected her career towards marketing, with a particular emphasis on scientific writing. Currently, as the Marketing Manager at Dynamic42, she specializes in Organ-on-chip technology.
About Dynamic42 GmbH
Dynamic42 GmbH was founded in 2018 and is a spin-off from the Integrated Research and Treatment Center for Sepsis Control and Care (CSCC) of the University Hospital Jena. Dynamic42 markets and develops human Organ-on-chip models/ microphysiological systems with integrated immune system components for research and testing of pharmaceutical products, novel therapies such as nanoparticles, chemical and food additives. The innovative organ-on-chip specialist just launched their new DynamicOrgan System and Developers Kit.
Credit : Dr. Juliane Fischer, Dynamic42 GmbH, iStock amgun