CHANGE : accelerate the effective use of NAMs in regulatory toxicology, Model Master Files, Literature for animal testing alternatives, and more
News on non-animal methods
NEWS, REPORTS & POSITION STATEMENTS
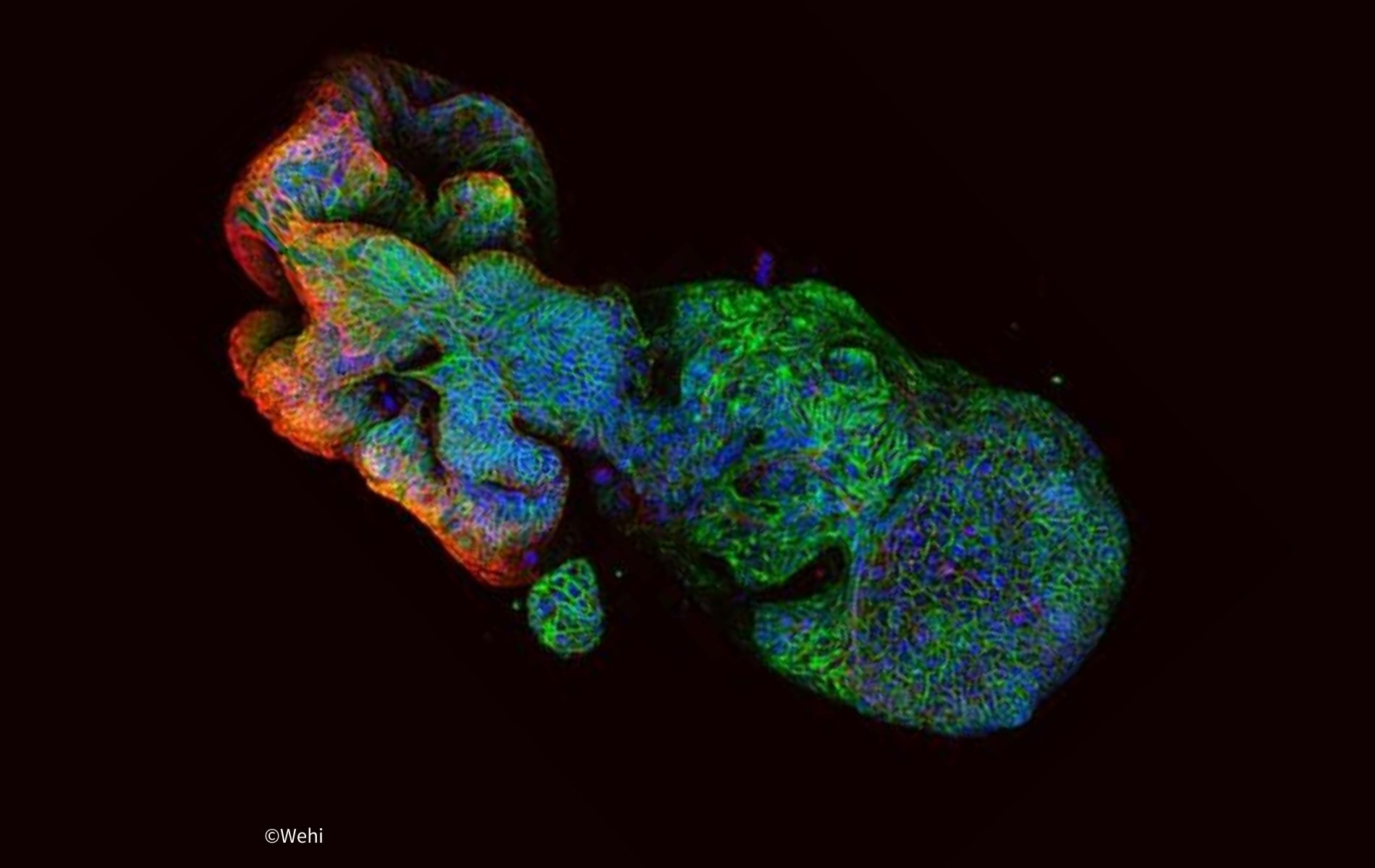
1. Lab-grown tumours predict treatment outcomes in landmark study
Bowel cancer, also known as colorectal cancer, remains the second leading cause of cancer-related deaths worldwide. A world-first study, led by WEHI researchers and published in Cell Reports Medicine, found drug testing on 3D patient-derived tumour organoids could predict how patients with advanced bowel cancer will respond to treatment with about 85% accuracy.
A clinical trial is now being developed based on the results, which will be the first to validate organoid drug testing as an accurate way to guide treatment selection for people living with bowel cancer – the second-deadliest cancer in Australia. “Our findings show that organoid drug testing is a potential game-changer for cancer treatment, suggesting the possibility of revolutionising personalised medicine and clinician-patient care through improved treatment selection.” — says Prof Gibbs, WEHI Laboratory Head.
Read the Publication in Cell Reports Medicine
2. Time for CHANGE : bringing forward the date of effective use of NAMs in regulatory toxicology
In a new article, authors introduced the “Collaboration to Harmonise the Assessment of Next Generation Evidence” (CHANGE) project, a new initiative that seeks to design system-level interventions for bringing forward the date of effective use of NAMs, explaining its goals, approach, project management, governance, and funding. CHANGE is far from the first effort to shape the use of NAMs in regulatory toxicology, and seeks to build on or complement efforts by ECHA, EFSA, ICCVAM, NTP, PARC, US EPA, and many others, all of which seek to inform, progress, and advance use and adoption of NAMs.
What makes CHANGE a unique contribution to the challenge of NAM uptake are : An emphasis on effective use of NAMs, a longitudinal, iterated, and interdisciplinary methodology, a focus on “system factors”, and an ambition of inclusivity and a global outlook.
3. Model Master Files (MMFs): The future of modeling for drug development
The world of drug development is rapidly evolving, and modeling and simulation (M&S) techniques are playing an increasingly vital role. As we strive for greater efficiency, cost-effectiveness, and patient access to innovative therapies, a groundbreaking concept has emerged – Model Master Files (MMFs).
MMFs represent a paradigm shift in how we approach and utilize quantitative models in the regulatory landscape. An MMF is a quantitative model or modeling platform that has undergone rigorous verification and validation, earning recognition as a sharable intellectual property acceptable for regulatory purposes. Stay tuned as the landscape of MMFs continues to evolve.
INTERVIEWS, NOMINATIONS & AWARDS
4. Revolutionizing chemical safety : PrecisionTox leads the change
The PrecisionTox consortium, an innovative European Commission funded project to transform regulatory toxicology, held its third annual meeting in Brussels on June 13 – 14. The gathering brought together leading scientists and legal experts from Europe and North America, who are all committed to enhance human health and to protect the environment from toxic substances.
On this occasion, Morven Pennie from the University of Birmingham won the prize for best poster ‘Daphnia Magna Monitoring System’.
TOOLS, PLATFORMS, CALLS
5. OECD 331 Guidance document on PBK : Feedback survey
The OECD Secretariat is interested to receive feedback on the OECD 331 Guidance document on the characterisation, validation and reporting of Physiologically Based Kinetic (PBK) models for regulatory purposes.
The guidance document has been published in early 2021 and has raised a lot of attention. Recently, the OECD received some feedback that could be included in a revision of the document. However, before starting this revision the OECD would like to understand from the Scientific and Risk Assessment communities the applicability, limitations, and issues that they have encountered when applying this guidance document.
The survey comprises 9 questions and take approximately 5 to 10 min to complete.
6. Searching the literature for animal testing alternatives
Tutorial
Searching for articles and other published literature on a topic is a multi-step process. A vast amount of published information exists for researchers looking for ways to reduce, refine, or replace their use of animal subjects in research. The University of North Carolina at Chapel Hill’s library system put together a Tutorial for researching alternatives to animal research.
Tools
A new National Toxicology Program webpage will allow users to easily search PubMed for information on specific topics related to alternatives to animal testing. NICEATM and the U.S. Department of Agriculture National Agricultural Library’s Animal Welfare Information Center (AWIC) collaborated to develop search strategies, known as “hedges” for topics specific to alternatives, available on their website. Additional hedges are under development ; and the center is open to suggestions of additional topics. AWIC developed search hedges for a number of animal welfare topics, and offers guidance and assistance for literature searches on animal use alternatives.
INDUSTRY, BIOTECH & PARTNERSHIPS
7. Queen Mary University of London secured £1.7 million to revolutionize drug discovery with OoC technology
The current drug development pipeline is plagued by high failure rates at the clinical trial stage. This is largely due to the limitations of traditional pre-clinical testing methods, often reliant on animal models that poorly translate to human biology.
A world-class team of bioengineers and scientists led by Queen Mary University of London, alongside GSK, UCB-Pharma, Emulate Inc, CN-Bio, Mimetas and the UK Medicines and Healthcare products Regulatory Authority (MHRA), has secured a £1.7 million research grant from the Engineering and Physical Sciences Research Council (EPSRC) to develop a groundbreaking approach to drug discovery.
8. Promising results in simulating effects of a drug on synthesized digital twin
Significant improvements in drug discovery and development can be made possible through in silico drug simulations using digital twins, by mimicking how a drug will interact with an individual patient’s unique biology, down to the cellular level to more accurately predict how drug compounds will behave prior to human trials, thereby reducing costs and failure rates.
GNQ Insilico, an AI-biotechnology company, simulated the pharmacokinetics and pharmacodynamics of an existing infertility treatment on thousands of digital twins, simulating outcomes to identify optimal dosing strategies tailored to each digital twin’s characteristics.The results highlight how genomics and AI can be used by the pharmaceuticals and life sciences industries to improve the efficiency of clinical trial designs for new drug development.
SCIENTIFIC DISCOVERIES & PROTOCOLS
9. Organoids for functional precision medicine in advanced pancreatic cancer
Patient-derived organoids (PDO) are promising tumor avatars that could enable ex vivo drug tests to personalize patients’ treatment in the frame of functional precision oncology (FPM). Yet, clinical evidence remains scarce.
The largest prospective study aiming at implementing PDO-based FPM ran by the French Gustave Roussy Institute’s spin-off Orakl Oncology, recently identified very robust predictive values in this clinical setting. In a clinically relevant turnaround-time, they identified putative hits for 91% of patients, providing unexpected potential survival benefits in this very aggressive indication. While this remains to be confirmed in interventional precision oncology trials, PDO collection already provides powerful opportunities for drugs and combinatorial treatment development.
10. Recent trends in anti-cancer drug discovery by in silico methods
Intensive drug discovery efforts have focused on the modulation of cancer-related molecular pathways, particularly those involving proteins with altered function or expression, also laying the groundwork for personalized medicine. In this context, computational approaches have greatly supported the drug discovery process, in some cases representing the driving force behind the discovery of novel small molecule therapeutics.
In a recent Research Topic, scientists collected valuable contributions showcasing success stories in the field of anticancer drug discovery and highlighting the synergies of transformative in silico approaches with traditional drug discovery, holding great promise for advancing cancer treatment and personalized medicine.
11. Most drugs tested in animals don’t progress to human approval
An additional new study has shed light on just how few treatments tested on animals are approved for use in humans. Among all of the 122 systematic reviews the team analyzed, only 5% made it to regulatory approval by the US Food and Drug Administration or health authorities in the UK or Switzerland.
Therapies for circulatory system disorders and mental health disorders fared the worst, with 1% and 0% of the treatments in the reviews reaching approval, respectively. Treatments for cancer and musculoskeletal diseases fared better, with 20% and 15% reaching approval.Two of this study’s authors both agree that animal trials aren’t going anywhere anytime soon.
Read the publication in PLOS Biology


