Using AI to Treat Breast Cancer, Clinical trials in-a-dish, NAM Journal, 3D bioprinting to Create Synthetic Blood Vessels and more
News on non-animal methods
OCTOBER 07 - 11, 2024NEWS, REPORTS & POSITION STATEMENTS
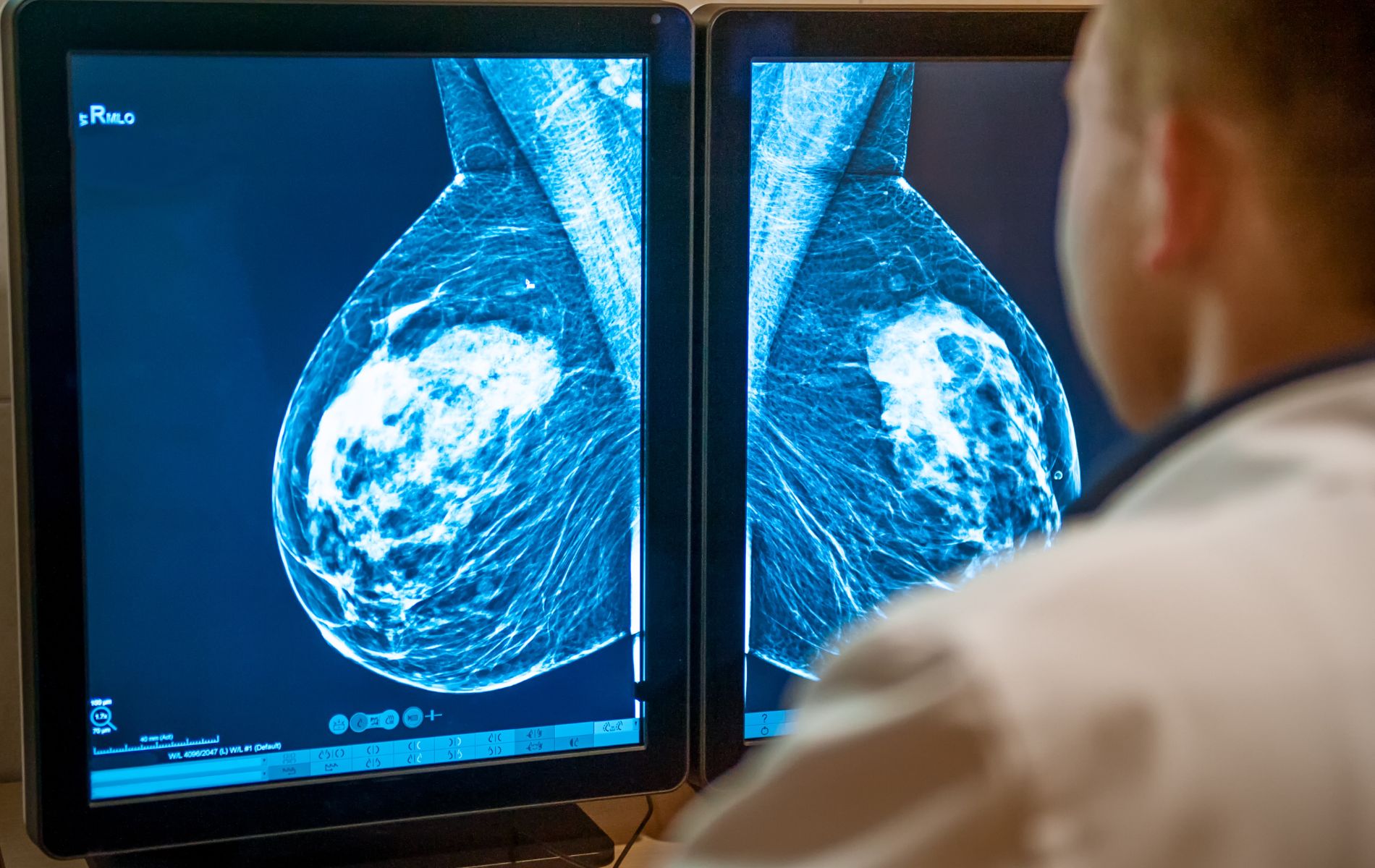
1. Using artificial intelligence to treat breast cancer
“Pink October” is Breast Cancer Awareness Month. Every year in France, 12,000 women die from this disease, while 61,000 patients discover they have it. But artificial intelligence is revolutionizing certain types of treatment. At the Institut Curie, as in all cancer centers, imaging is omnipresent. From simple mammograms to the most sophisticated MRIs, these images are essential for oncologists in adapting their treatments.
Working with the manufacturers of these machines, the researchers were able to teach the AI how to predict a patient’s future : her survival rate, possible complications, and so on. Several clinical trials are already underway to validate these predictions, and it’s already safe to say that tomorrow, AI will know before doctors and patients do what the patient’s response to the chosen treatment will be. Tara Schlegel reports.
Read more and listen to the podcast (FR)
2. Clinical trials in-a-dish for cardiovascular medicine
Cardiovascular diseases persist as a global health challenge that requires methodological innovation for effective drug development. Conventional pipelines relying on animal models suffer from high failure rates due to significant interspecies variation between humans and animal models. In response, the recently enacted Food and Drug Administration Modernization Act 2.0 encourages alternative approaches including induced pluripotent stem cells (iPSCs). Human iPSCs provide a patient-specific, precise, and screenable platform for drug testing, paving the way for cardiovascular precision medicine.
A new review in the European Heart Journal discusses milestones in iPSC differentiation and their applications from disease modelling to drug discovery in cardiovascular medicine. It then explores challenges and emerging opportunities for the implementation of ‘clinical trials in-a-dish’. Concluding, the review proposes a framework for future clinical trial design with strategic incorporations of iPSC technology, microphysiological systems, clinical pan-omics, and artificial intelligence to improve success rates and advance cardiovascular healthcare.
3. New NAM Journal
NAM Journal embraces these recent advancements by serving as a hub for dissemination and worldwide exchange of information regarding state-of-the-art NAM developments. NAM Journal welcomes original research papers, review papers, opinion papers and meeting reports dealing with NAM production and their use in toxicology, chemical risk assessment and biomedical research, amongst other fields. Manuscripts can address all aspects of the NAM life cycle, from conceptual development to acceptance, implementation and application.
NAM Journal is a 3I (international, interdisciplinary and intersectoral) journal and encourages submission from all relevant disciplines, ranging from natural, formal, life and applied sciences all up to social sciences and humanities. The journal target audience includes undergraduates to full professionals in academic, industrial and regulatory settings in any part of the world.
4. Minimum information for reporting on the TEER assay
A new paper out in Archives of Toxicology, describes how to standardise testing to support data transparency and reproducibility, enable cross-laboratory comparisons, and facilitate the incorporation of the Transepithelial Electrical Resistance (TEER) assay into national and international testing guidance. While the recommendations focus on respiratory epithelial cell systems, these recommendations can be adapted for other cell systems that form barriers.
The paper was co-authored by PETA Science Consortium International e.V. and twenty-two other organisations, including the US Environmental Protection Agency (EPA), the Luxembourg Institute of Science and Technology, Epithelix Sàrl, the Jai Research Foundation, the Clorox Company, the Battelle Memorial Institute, and BASF SE.
TOOLS, PLATFORMS, CALLS
5. Documentary Project : Is biomedical research at a turning point ?
Is biomedical research at a turning point ? Innovative technologies such as multi-organ chips, AI, 3D bioprinting and computer-aided models have the potential to replace animal testing while providing more precise and faster results for humans. But despite these promising developments, numerous animals are still used in research. Are animal testing really indispensable for medical progress ?
FUTURE SCIENCE wants to make this important topic accessible to a wider public in a 90-minute documentary film in order to draw attention to the importance of animal-free research. The goal is to initiate an open discussion and show how crucial this change is for future-oriented research. The film is intended to raise awareness and pave the way to a future free of animal testing.
6. NIH funding for OoC in dental, oral, and craniofacial research
This funding opportunity encourages interdisciplinary research that advances the validation of organ-on-a-chip (OoC) towards disease modeling and pre-clinical efficacy studies in dental, oral, and craniofacial (DOC) research.
It is expected that outcomes will advance the use of validated reproducible three-dimensional microfluidic systems into the framework of clinical trials with demonstrated usefulness as new approach methodologies for DOC clinical research. An essential feature will be a multidisciplinary approach including experts in DOC biology and clinical science, pathology, microfluidics, bioengineering, material science, computational biology, pharmacology, and biostatistics.
Deadline : October 19, 2024
INDUSTRY, BIOTECH & PARTNERSHIPS
7. Owkin INVOKE drug passed US FDA Phase 1 for investigational immuno-oncology
INVOKE (OKN-4395 – 121) is a biomarker-rich Phase 1, open-label, multicenter, dose-escalation and cohort expansion study of OKN4395, a dual antagonist of EP2 and EP4 prostanoid receptors, as monotherapy and in combination with pembrolizumab, in patients with advanced solid tumors that are considered as unmet needs.
Scheduled to open for enrollment in the US, UK, and Australia in early 2025, the study represents the first major undertaking for Owkin’s in-house clinical development, leveraging its operating system OWKIN K OS v1 — towards biological Artificial General Intelligence (AGI). For now, Owkin K OS v1’s most important impact for the program is to discover the right indications to target where they believe biology can be homogeneous.
Owkin also just announced a partnership with AstraZeneca to develop an AI pre-screen solution for BRCA genes in breast cancer.
SCIENTIFIC DISCOVERIES & PROTOCOLS
8. Multiple sclerosis : a gene reading defect identified in patients
Multiple sclerosis (MS) is an inflammatory disease in which the immune system attacks the central nervous system. The molecular mechanisms behind the disease remain poorly understood. In an article published in Life Science Alliance, scientists reveal how loss of control of the enzyme that reads genes may explain several aspects of the disease.
By analyzing rare RNAs, the study shows that, in certain MS patients, a deregulation of gene expression in the immune cells responsible for destroying pathogens is linked to dysfunctions in the Integrator complex, a protein machinery essential for maturing non-coding RNAs.
Read more (FR)
9. Human trophoblast organoids for improved prediction of placental ABC transporter-mediated drug transport
The disposition and drug-drug interaction of clinical drugs are closely related to the functions of ATP-binding cassette (ABC) transporters. The trophoblast is a unique feature of the placenta, which is crucial for normal placentation and maintenance during pregnancy. Due to the lack of appropriate modeling systems, the molecular mechanisms of regulation between ABC transporters and trophoblast remains unclear.
In this new report, trophoblast organoids were cultured from human placental villi and developed into three-dimension structures with cavities. Trophoblast organoids exhibited transporter expression and localization comparable to that in villous tissue, indicating their physiological relevance for modeling drug transport. Two commonly used hypertension drugs and three antipsychotics were chosen to further validate this drug transport model.
10. Engineers apply 3D bioprinting to model deadly brain tumors
Glioblastoma is a brain cancer with very poor survival outcomes. Most drugs cannot cross the blood-brain barrier, which means that unlike other cancers, there just aren’t that many therapies available for brain tumors. A cutting-edge technology developed at the University of Cincinnati aims to change that.
Researchers are using 3D bioprinting to create artificial blood vessels that can be used to test new custom-tailored drugs and study why glioblastoma is so resilient. Using standard technology, organs on a chip can take months to make. But Dr Barrile and his students can use 3D bioprinting to create custom synthetic blood vessels far more quickly for each patient.
Read the publication in Advanced Healthcare Materials
11. In Vitro prediction of skin-sensitizing potency using a simple regression approach
Toxicological assessments of skin sensitizers have progressed towards a higher reliance on non-animal methods. Current technological trends aim to extend the utility of non-animal methods to accurately characterize skin-sensitizing potency. The GARDskin Dose – Response assay has previously been described ; it was shown that its main readout, cDV0 concentration, is associated with skin-sensitizing potency.
A new study further characterize the GARDskin Dose – Response assay and provide results that show that its readout, the cDV0 value, correlates significantly with established potency metrics on a larger dataset and can be used to accurately predict potency. Notably, the redundancy and potential ambiguity of having repeated No Expected Sensitization Induction Levels (NESILs) derived from the murine Local Lymph Node Assay (LLNA) and human No Observed Effect Levels (NOELs) for model fitting was rectified using a composite potency value describing a latent potency signal.


