
EXADEX-INNOV: Shaping the future of preclinical research ex vivo
Dr Vincent Dani
SES114 — October 2024Towards more clinically relevant models
Vincent Dani is co-founder of the French start-up ExAdEx-Innov, which develops innovative, patented technologies based on human adipose tissue. In addition to his skills as a deeptech entrepreneur, Vincent has a PhD in Cellular and Molecular Biology from the Université Côte d’Azur and training in clinical research management (CRA training at the Faculté de Médecine de La Timone, Marseille).
In this interview, Vincent Dani, gave us the honour to dive into the particularities of his company, unique in the world, and its technological process for developing ex vivo human adipose tissue derived from patients.

Pro Anima Scientific Committee: We had the chance to talk for the first time in October 2023, and we discovered your fantastic work. Before going into more detail about the specificity of your research, could you introduce yourself to our readers and tell a bit more about your career path leading up to the launch of ExAdEx-Innov?
Dr Vincent Dani: I’ve had a fairly classic academic career: I obtained my PhD in 2015 in cellular physiology and molecular biology, then went on to do several postdoc focusing on basic research, always with the desire to use the knowledge acquired in the academic world to develop practical applications that are useful to our society. In 2018, I joined the Stem Cells and Differentiation laboratory at the Valrose Institute of Biology in Nice as a research engineer. My project, supported by an organisation that funds academic projects with market applications, used new cell culture techniques to develop 3D models from human adipose tissue. We very quickly succeeded in developing a process that enabled us not to produce in vitro 3D cell models, but to directly culture human adipose tissue ex vivo for several weeks.
This approach was quickly patented, in 2019, with the support of the Sociétés d’Accélération du Transfert de Technologies (SATT), and we began to receive requests from the market, from companies that saw concrete applications with our method for the development of their products or medicines.
In 2022, we decided to create EdAdEx-Innov. We are five co-founders, myself and Luigi Formicola, a surgeon and two academic team leaders. I co-direct the company, particularly the scientific side, and my colleague Luigi is in charge of development, market and strategy.
ExAdEx-Innov is incubated in the academic laboratory where the technology was developed, on the Faculty of Medicine campus. What is stimulating and quite original is that we are a private company in the middle of an academic and hospital environment, which means we can still do a lot of research and development.
Pro Anima Scientific Committee: Can you explain ExAdEx-Innov’s technology, and how this platform of adipose tissue derived from human patients is innovative and unique in France and even in the world?
Dr Vincent Dani: The patented technology is surprisingly simple, in the sense that it doesn’t require any special equipment. However, it resulted from more than 30 years’ experience and knowledge of the research teams in the laboratory where it was developed. It is a process that enables us to recover adipose tissue, and therefore fat, directly from donors in the form of liposuction or abdominoplasty.
Using this ‘surgical waste’, which is normally eliminated by the medical profession, we have succeeded in developing a process that enables the tissue to be cultured ex vivo, while preserving the physiological responses via the microenvironment, the vascularisation, the matrix and the immune cells.
Eight weeks is the limit we have chosen not to go beyond, because after a while the physiological characteristics start to become less stable, or even decline [1]. In fact, we don’t have a classic laboratory model that stabilises over time, but rather a model without exogenous contributions, and therefore without bias, that retains the memory of the donors. This is true for the belly fat, face fat and thigh fat that we work with.
Using this preserved tissue, we can carry out fundamental research to understand how this adipose tissue functions, or offer this technology to companies wishing to develop products, medicines or even new techniques without having to use animals or organoid models. The human fat model is much more physiologically and clinically relevant because of its complexity. Our process enables us to work at the tissue level, which is the key point of this innovation.
The technological challenge we have overcome is not to dissociate the fat using enzymes, which would lead to cell culture, but to do it mechanically using small reducers. This way, we obtain structures that are smaller than the normal order of several millimetres. The mechanically ‘attacked’ tissue will regenerate to a size that is functional and compatible with culture and media exchange. What we often see with increasingly large and complex 3D cell models is that the culture medium does not penetrate into the inside, leading to necrotic bodies (clusters of dead cells) [2].
We can work in various conventional cell culture containers, either in low-adhesion conditions adapted to 3D culture, or in adherent conditions using an agarose encapsulation method to recover conventional culture conditions, while maintaining the conditions of a 3D suspension culture.
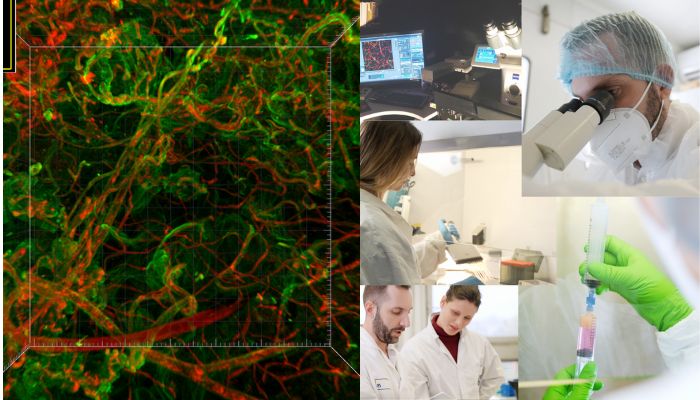
Pro Anima Scientific Committee: Speaking about cell culture media, Pro Anima is particularly interested in biomaterials and animal-derived products, particularly the addition of foetal calf serum, which is very commonly used. Do you have a well-defined culture medium that is better suited to human cells?
Dr Vincent Dani: Absolutely. That’s a very good question. We were initially working with a commercial medium that promotes vascularisation, a medium designed for endothelial cells. As we are also working on biotherapy projects, we need to have well-defined media and to know each product making up the medium, composition and concentrations.
We have worked to reduce the number of different compounds in the medium as much as possible, each time empirically trying to remove one compound after another, enabling us to reduce the culture medium to the minimum necessary for the tissue to maintain itself. In this way, we were able to grow serum-free adipose tissue, the characteristics of which are more or less comparable to those obtained with the addition of serum. The biological explanation we put forward is that this is a fairly complex human tissue, with numerous cell types that secrete their own growth factor.
We developed these serum-free media for biotherapy, but also at the request of certain customers in the cosmetics industry who wanted an animal-free cell culture. That said, we don’t use serum-free media all the time, because for the time being, it always works better with serum. However, we do have the capacity to do this without serum if regulations were to prohibit the use of animal serum in the future, or in the event of shortages — and this is a real topical issue — in case we run out at some point. On a more fundamental level, the fact that we are able to remove as many compounds as possible, including serum, is part of our approach/logic of getting as close as possible to what happens at the level of human physiology, both to meet market demands, with a view to reduce animal inputs, and also for biotherapy, where the regulatory level demands that there is no input of animal products. Our approach is therefore based on both regulatory and ethical considerations.
Pro Anima Scientific Committee: Animal testing for cosmetics has been banned in France and Europe since 2013. Do you have many interactions with players in this field and success stories with your platform that you’d like to share with us?
Dr Vincent Dani: Yes, absolutely. The cosmetics market was the first to take an interest in our models, not least because we developed our models using facial fat, which was even more interesting than abdominal fat, particularly for dermocosmetics. Facial fat models are much more physiologically and clinically relevant for these players, as the majority of the products they develop are for facial applications, with anti-dark circles, anti-wrinkles, etc.
The message we heard from these companies, which carry out a lot of clinical trials by testing their final products on human panels, was that the cellular models developed so far were no longer sufficient. The limitation of cellular models is that they do not include all the different layers and other components of the skin. It is important for them to be able to test on models that are as close as possible to the final application at the early stages of development. Our tissue models retain the vascular and lymphatic networks, and all the matrices including the proteins normally present in human adipose tissue.
Cellular models are very good for everything to do with toxicity, but they are less relevant for demonstrating the potential of active ingredients. In this respect, we have a very good example of ExAdEx-Innov’s collaboration with an industrial company based in Monaco that develops active ingredients (based on silicon). Using our adipose tissue models, we tested the effect of cosmetic active ingredients at the overall tissue level, looking at the effects on the matrix, vascularisation and lymphatic network, and not just on the cellular part of the tissue. This work will be presented at the International Cosmetics Congress to be held in Brazil in 2024. This is a fine example both industrially and commercially, with a company that wants to build scientific stories behind its products, demonstrating that using models that are more complete and closer to humans makes it possible to identify effects at a global level.
This example also shows that alternative models, such as those developed by ExAdEx, are beginning to be visible at scientific conferences, not (solely) as an alternative to the animal model, but because they are more consistent with human physiology. This need for performance is also important in view of the economic stakes involved.
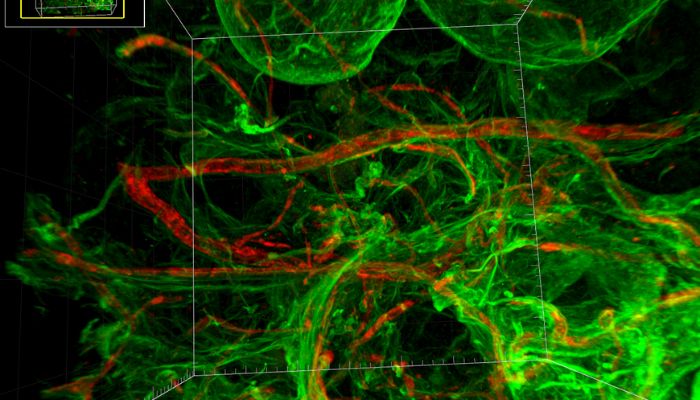
Pro Anima Scientific Committee: What is your relationship and experience with the private sector, academia and regulatory agencies? In what areas other than cosmetics can your approach also have concrete applications and replace animal models?
Dr Vincent Dani: The fact that we are based in an academic research community means that we still have a lot of interesting projects with university hospital centres, and can collaborate with basic research or clinical research, using these more physiological models.
ExAdEx works a lot with aesthetic and regenerative medicine, but also with pharma groups that are starting to take an interest in our technology, particularly with the adoption in the United States of the FDA Modernization Act 2.0, which paves the way for the development of drugs without necessarily going through a step with animal models. Canada is likely to do so soon, and let’s hope that this will give Europe some good ideas for pursuing this.
One pharmaceutical area where the development of new methods is consequent is treatments for obesity and metabolic diseases. Numerous scientific publications have shown that animal models, in particular mice, have a metabolism or distribution of fat mass that is totally different from humans [3]. Our human adipose tissue models, which summarise the differences in the population in terms of body mass index and metabolism, are more clinically relevant, with responses that are unbiased, unlike what you would get in animal models.
There are many drugs that show positive results in mice but, because of different metabolic pathways and fat distribution, have no effect when tested in humans.
Even if animals can still be used today, there will be a real interest in no longer using them, not because regulations will demand it, but rather because new technologies are more relevant, quite simply. These are much more than alternative models. We have a greater interest in showing pharmaceutical companies and also regulators that these models are not just alternatives, but that they are better and can provide more reliable and more clinically representative answers. If this is demonstrated across the board, the use of animal experiments will be reduced.
And this will be more likely to happen at European level. In the United States, there seems to be a large community, or one that is growing, around microfluidic models (MPS) and their validation. The same thing is starting to happen at the European level. More generally, organisations like the CEA, which are very active at the European level, are going to push things forward in this area.
Pro Anima Scientific Committee: During our first discussion, you mentioned problems of access to tissues and/or associated databases. Is this still the case today? What solution(s) do you think could be put in place in France or Europe to alleviate the problem?
Dr Vincent Dani: I’m going to answer the question in two parts.
We are working with our co-founder, Dr. Chignon-Sicard, to build a network of surgeons, with the support of the CHU de Nice. Thus, we have access to human material via donors who agree to make available what we call their “surgical waste”. This gives us access to more or less controlled sourcing. We are also working with the University Hospital in Nice to submit applications for clinical trials, thereby gaining access to adipose tissue from patients, this time obese, to develop research projects.
What we are also seeing with ExAdEx-Innov’s collaborations, and with our presence within research consortia, is that having access to clinical databases or human material catalyses significant progress in research. These data are representative of the differences within the population, and therefore make it possible to answer more specific and tailored questions.
On our side, we are working on frozen tissues without losing physiological capacity, with the ultimate aim of developing human tissue banks. For the time being, this is not something we have brought in-house, because there is a whole regulatory authorisation process involved in setting up a patient-derived bank. It remains quite difficult because in France access to human material is highly codified and regulated. This is good and reassuring for patients and donors, as it guarantees the quality of the research. On the other hand, because the regulatory framework is so demanding, the process is lengthy.
The solution, however, may be fairly simple: increase the resources allocated to certain bodies, and more specifically to the Personal Protection Committees (CPP), which are responsible for issuing a prior opinion on the conditions for the validity of any research involving human material.
There are two ways of obtaining surgical waste from patients: in the case of a person who comes in for liposuction, their biological material is normally disposed of by the surgeon and does not require any further surgery. With the donor’s consent, the material can be recovered through only minor administrative requirements. If human material is to be recovered as part of an operation where this tissue is not normally disposed of, the surgeon must perform an additional operation. In such cases, it is necessary to go through clinical trial applications managed by committees at the ANSM (Agence Nationale de Sécurité du Médicament et des Produits de Santé) and the CPPs. These CPPs will assess the relevance of the project, its scientific and ethical interest, the risk to the patient of having to undergo a deviation from the operation for which he or she normally comes, and whether or not there is no risk to the patient. This risk is the first criterion. If there is no risk, the CPP will assess the relevance and scientific impact of the project that is the subject of the application.
The CPPs members are researchers and professionals, who are certainly still involved in research activities and maintain a high level of expertise, but who are volunteers and receive a large number of applications. These assessments take time. This time, which is necessary, is also a guarantee that biological material from donors or patients is used in projects that are really worthwhile, so that future donors can be confident about the use of their material.
Allocating more resources to the CPPs would accelerate the authorisation process and decision. To illustrate, our request to access tissue from obese patients for bariatric surgery took almost a year, which is a very long time.
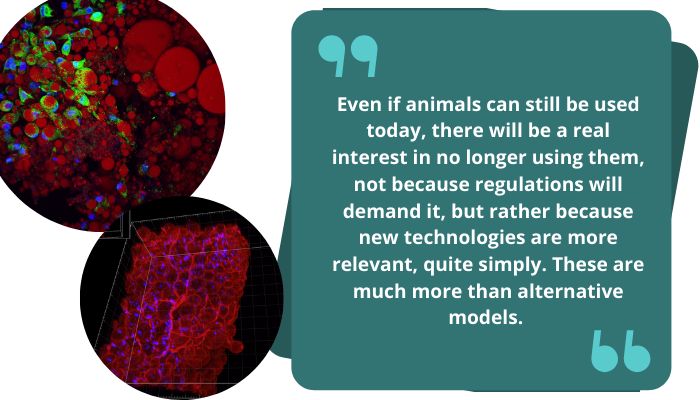
Pro Anima Scientific Committee: Have you encountered any technical or social obstacles in the development of your start-up?
Dr Vincent Dani: No, not necessarily. As we come from university, we have received a lot of support at the local level, from the Côte d’Azur University and its Idex excellence programme, and from technology transfer support structures, whether incubators or health clusters. Support for scientific and disruptive innovation in France is fairly well organised. If you talk to other entrepreneurs or researchers at European level, the French system is in a pretty good position. Among other grants, we have benefited from the French Tech grant from BPI France.
So I would say that there aren’t really any major obstacles to the development of companies offering scientific and disruptive innovations. After that, the success and longevity of this type of company depends on its ability to find a real demand from the market or the scientific world in general. So, in our case, the brakes are not really social, but more related to science, which will tell us whether what we have developed is of interest or not. This is the particularity of our companies, which the BPI describes as deeptech; because we are developing a disruptive innovation, we are shaping the future of tissue models. As we are the first to develop these adipose tissue models, we are in a way creating the market, the demand, and we are trying to show that these models are more relevant.
Pro Anima Scientific Committee: Would you be willing to share with us one or more projects in development and your long-term vision for your start-up and for biomedical research more generally?
Dr Vincent Dani: We have touched a bit about it throughout this discussion, our aim at ExAdEx is to develop models that are closer to human physiology.
With this in mind, we recently received funding from the French National Association for Research and Technology (ANRT) to recruit a doctoral student under a CIFRE contract, whose aim is to work on gaining a better understanding of how the adipose tissue of obese patients functions.
It is very hard to obtain pathological adipose tissue from obese patients. So the solution we want to develop is to recreate this pathological environment with healthy tissue obtained from surgical waste. We are working in collaboration with a research team specialising in advanced sequencing techniques to really understand how the pathology works. This will enable us to recreate appropriate research models and test molecules and drugs in a physio-pathologically relevant environment.
Our doctoral student started last November. The initial results, which I’m sharing with you exclusively, are that we have succeeded in reproducing in the laboratory virtually the same inflammation that we observe in the adipose tissue of obese patients, using samples of surgical waste from ‘healthy’ people.
The next step is proposing models that recreate not the normal environment of a tissue, but different functional and physiological states. Obesity is associated with metabolic diseases, in particular fatty liver disease, where the pathological adipose tissue secretes a certain number of compounds that put the liver into an inflammatory state. We are starting to develop models to understand the interactions between adipose tissue and the liver. We are developing this in-house, but we can also develop it with leading-edge organisations in the field of microfluidics.
This is not an alternative to experiments on mice that have been made obese, where the mechanism is completely different. A mouse does not become obese in the same way as a human. We are not replacing animal models for ethical reasons, but for biological reasons. What is most encouraging is that the companies that come to work with us are looking for development through science. It is not just about having an alternative model, but really about trying to understand mechanisms better by having models that are closer to clinical reality, doing more science and making science more relevant. The way we work and develop is really driven by the market, which wants to be innovative and find solutions. It is not just driven by marketing and sales. It comes from both sides. That is why it is so stimulating.
References
[1] “Regulation of Adipose Progenitor Cell Expansion in a Novel Micro-Physiological Model of Human Adipose Tissue Mimicking Fibrotic and Pro-Inflammatory Microenvironments”, Vincent Dani et al. Cells 2022 Sep 7;11(18):2798.
[2] « Engineering organoids » Moritz Hofer and Matthias P. Lutolf, Nature Reviews Materials volume 6, pages402 – 420 (2021)
[3] « Of mice and men: Considerations on adipose tissue physiology in animal models of obesity and human studies » Ioannis G. Lempesis et al. Metabol Open. 2022 Sep; 15: 100208
Read More
ExAdEx-Innov
Unique R&D platform of donor-derived real human adipose tissue ex vivo for pharmaceutical drug development (obesity, chronic metabolic diseases), dermo-cosmetic, nutraceutical, aesthetic and reconstructive surgery research.
ExAdEx-Innov owns exclusive worldwide rights to ExAdEx patented breakthrough technology from French academic laboratories of excellence (Côte d’Azur University, Institut de Biologie Valrose, CNRS, INSERM, University Hospital of Nice) and is supported by SATT Sud-Est, IdEX Université Côte d'Azur, CNRS Innovation, Provence Côte d'Azur Business Incubator, Métropôle Nice Côte d’Azur and Eurobiomed Healthtech Accelerator.
Credit : ExAdEx-Innov



