
Prof. Donald E. Ingber
Disrupting drug discovery and testing with human Organs-on-Chips
SES112 — March 2024Donald E. Ingber is the Founding Director of the Wyss Institute for Biologically Inspired Engineering at Harvard University, Judah Folkman Professor of Vascular Biology at Harvard Medical School and Hansjorg Wyss Professor of Bioinspired Engineering at Harvard School of Engineering and Applied Sciences. He received his degrees (B.A., M.A., M.Phil., M.D. and Ph.D) from Yale University. Ingber is a pioneer in the field of biologically inspired engineering, and his most recent breakthrough is the development of human Organ-On-Chip microfluidic culture devices lined by living human cells that are being used to replace animal testing for drug development, disease modeling, and personalized medicine. At the Wyss Institute, he and his teams have made great strides in translating their innovations into commercial products to advance healthcare or to improve sustainability and many are now either in clinical trials or currently being sold. Ingber has authored more than 500 publications, 200 patents, and founded 8 companies, including Emulate Inc., the leading manufacturer of Organ Chip systems.

Comité Scientifique Pro Anima : Can you kindly give us an overview of who you are and how you became the founding director of the prestigious Wyss Institute ?
Pr. Don Ingber : I am Don Ingber, founding director of the Wyss Institute for Biologically Inspired Engineering in Harvard, and a chaired professor at both Harvard Medical School and its School of Engineering, as well as at Boston Children’s Hospital for 40 years now. I have a Doctorate of Medicine and of Philosophy from Yale. I am a cell biologist PhD but I got into bioengineering very early on because of my interest and belief that mechanical forces are as important for biological control as chemicals and genes. Now, it is called mechanobiology. I soon realized I had to develop new technologies to demonstrate the central role of physical forces, and to collaborate with physicists, engineers, chemists, and computer scientists to fully address this challenge.
In 2005, I was asked by the provost of Harvard University to co-chair a committee to envision the future of bioengineering across the university and its affiliated hospitals. Engineering has transformed medicine and industry over the last 50 years by applying engineering principles to solve problems in these fields. Based on how much we have learned about how Nature builds from the nanoscale up, we felt that we could flip the paradigm by now leveraging biological principles to develop new engineering innovations. And that was the basic concept, what we call “biologically inspired engineering”. The institute, founded in 2009, is primarily a translation institute, aiming at having near-term impact. Not only do we make breakthrough discoveries and develop new technologies, but also we have developed a process for de-risking these technologies technically and commercially to accelerate their translation towards commercialization. One of our key measures of success is intellectual property creation. Our institute, with only about 12 core faculty members, is now responsible for over 20% of all of Harvard’s patents and startups every year. So it has really been an incredible adventure.
PA : Could you give us more insight on the organ-on-chip technology, how it was created and why it is relevant for human health discoveries ?
Pr. D. Ingber : Organs-on-chips (OOC) from our institute are small devices, basically the size of a computer memory stick, optically clear, made out of a flexible silicone rubber material. The device has tiny microchannels through which we flow fluids, hence the name microfluidics. Our devices have two hollow channels that are parallel with a porous membrane so we can create organ-level structures by having two different tissue types interfaced across the membrane. For example, we can have lung lining cells — the epithelial cells of your air sac — on top and the lung capillary blood vessel cells on the bottom to recreate the alveolar-capillary interface. We can flow air over the top like in the lung, and we add a blood substitute like culture medium or even blood on the bottom. Because mechanical forces are so important for organ development and function, we have side chambers where we apply a cyclic vacuum that stretches the walls of the middle chamber with the tissue interface, to mimic breathing motions in the lung, peristalsis in intestine, or physiological deformation of an organ. We can basically recreate the physical and the chemical microenvironment as well as the tissue-tissue interfaces and 3D structures that exist in the body on these little devices. I call the OOC “synthetic biology at the cell, tissue, and organ level because it is really flexible, we can control and vary every parameter individually and in a combination. And I think that is really the leap in terms of potentially replacing animal testing, because you cannot separate these control features in an animal. OOC are impressively able to mimic human physiology and disease states with higher fidelity than any other systems that are out there.
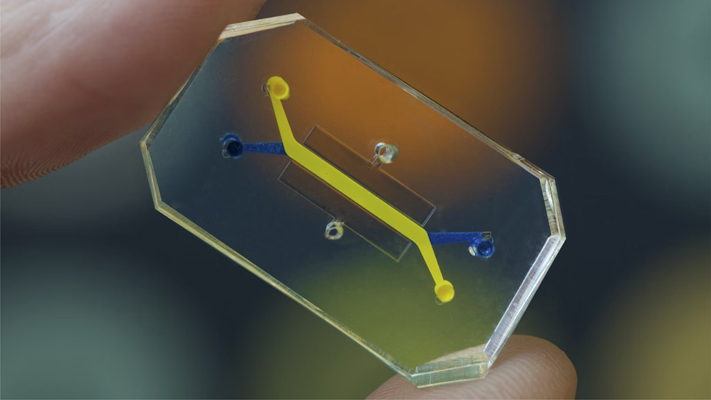
Image of an Organ-on-Chip — OoC
PA : We recently heard a lot about the success of Emulate work using liver-chips to predict DILI (Drug-Induced Liver Injury). Do you have other examples in mind showing that OOC can be much more predictive of human health than animals ?
Pr. D. Ingber : Many ! We published a paper where we developed a bone marrow chip. Astrazeneca, the pharmaceutical company, had a drug in clinical trials that showed a very unusual toxicity in the bone marrow. The same dose of this drug given over two hours versus two days had different toxicities. It was hard to mimic or understand it in an animal model. So they asked us if we could do it in our model. Because our chips have flow that passes through a channel lined by blood vessel cells and they have tissue-tissue interfaces, we can mimic how the drug levels change over time in the body, what is known as pharmacokinetics, which is absolutely critical for drug actions as well as toxicities. As you know, if you take a drug — your doctor says “take it once a day” — three times a day you can get sick or even die. In our bone marrow chip, we could precisely mimic the toxicities AstraZeneca saw in measuring the blood of their patients. Our chips flow the drug through the blood channel like in the patients (1). I can give you many more examples : we have used our lung chips to identify drugs that have moved to clinical trials for Covid-19 (2,3). And there are many more, but I think the bone marrow is a great example.

In those same bone marrow chips and that same publication, we made chips with cells from children who had a rare genetic disorder where they have abnormal bone marrow function. The bone marrow beautifully mimicked the phenotype that is seen in those patients, the same abnormality. We also discovered that there are two subpopulations of patients with this rare disease that have a particular differentiation defect nobody ever knew before. So we obtained new insight into the disease process. We have also been funded by the FDA for the last 12 years or more to use organ chips to study radiation toxicity because it’s very hard to do this in clinical trials and in animals the radiation dose sensitivity as well as phenotypes are different than in humans. When we irradiated the bone marrow chip, we observed the same dose sensitivity that we saw in humans and the same phenotypes.
I’ll give you one other example. We were funded by the Bill & Melinda Gates Foundation to do something that is a truly unique power of the OOC, which is to study complex human microbiome in direct contact with human cells and tissues. The microbiome has been a major paradigm shift in medicine in the last 20 or 25 years. We have living microbes co-inhabiting our bodies : in our gut intestine, our mouth, vagina, everywhere. And they are important for not only disease but health. The Gates Foundation has a project with kids in low resource nations who have a malnutrition related disease, called “Environmental Enteric Dysfunction” (EED). It is known that in addition to low nutrition, microbiome, immune response and other factors also contribute to the disease. So it is a multifactorial disease. We got cells from kids in Pakistan with EED and we made intestine chips with them. We actually can put healthy versus diseased microbiome from these kids on the chips, with or without malnutrition. And just using malnutrition alone on the chips, with a nutritionally deficient medium, we can mimic the transcriptional profiles of these kids (we compared with data obtained from intestinal biopsies from these children by clinical collaborators in Pakistan). Whereas that same nutritional deficiency with cells from healthy kids you didn’t see that. Now we have data — not yet published — that shows when you add the microbiome from EED kids to EED chips, you can see an additive effect that you do not see with healthy microbiome. So we are really getting human mimicry, clinical mimicry, that you cannot see in cultures or even in many animal models as animals have a different microbiome.
Organs-on-chips are impressively able to mimic human physiology and disease states with higher fidelity than any other systems that are out there
PA : Pro Anima has recently been part of the EU workshop to set up a roadmap for phasing out animal testing in chemical safety assessments, and it was clear that validation of alternative methods is one of the biggest challenges. In your opinion, what would be the best way to achieve regulatory acceptance ? Especially for OOC ?
Pr. D. Ingber : There is the Emulate study — that I was part of — where they tested 27 different drugs that were already known to be toxic to the liver or safe in humans and animals. They show that the human liver-chips are 7 to 8 times more predictive than animals in predicting liver toxicity and they were 100% accurate in predicting safety. This is the sort of qualification approach that needs to be done. Currently, regulatory agencies let companies propose what they want to use as validation criteria. Yet, when some criteria are proposed, the answer of the agencies is that it is not enough, which is a waste of time for everybody. So there is a need for more global harmonization of the criteria used for qualification of the techniques and validation of those criteria. And it would be enormously helpful for everybody if the FDA and European Regulatory Agencies come up with a common set of expectations.
However, the idea of a harmonization of one chip design is unrealistic. You have to have competition. Some systems may be better for certain applications, and some for others. What I do believe is critical is robustness, demonstrating that you can create robust systems so that different groups at different sites obtain the same results with the same chips, and working out manufacturing and cost. Agencies want these models to be qualified, confirming they are \ robust, that they faithfully mimic human level functionalities. We need to hit a point where processes and systems are automated enough and robust enough so that they really begin to get integrated into drug development pipelines. Even the FDA knows we need something better than animal models. But they need to be convinced that new models are as good or better. That is really what it is all about. And that requires data.
PA : The US has made a big step toward phasing in NAMs with the FDA Modernization Act 2.0. What are your views on this legislation and do you think it is enough to accelerate the replacement of animals in biomedical research and toxicology ?
Pr. D. Ingber : It is fantastic. I was involved with talking to the Congress. And it is amazing that this legislation now clearly states that FDA can consider data from OOC or other human relevant alternative methods instead of an animal model. But in a sense, that was always a possibility. For instance, Vertex got their drugs for cystic fibrosis into patients without ever using animal models, because they used human cells with a single gene defect. And that was enough to convince the FDA with those data.
However, it is much easier to use these systems for drug discovery and drug efficacy than it is for toxicology. There is a highest hurdle for taking a risk — and that is really what the regulatory agencies are focused on — toxicity. Also, when drug companies do liver toxicity in animals, they are not only looking at the liver but also the kidney and all the organs. So results with a single OOC is not going to convince them to replace animal testing. But because many times the drugs produce contradictory results in these animals and they do not know what to do with the results, I think Emulate’s liver chips could provide a powerful way to “rule in or rule out” whether a drug should progress further in the drug development pipeline, and hence narrow down drug testing. In that way it would reduce animals and more importantly, it would reduce failures later on. There are also good OOC models of kidney toxicity and heart toxicity. So you can begin to see how these could be used alone and together, and to progressively really reduce the animal numbers and maybe eventually replace them in the long-term.
What I do believe is critical is robustness, demonstrating that you can create robust systems so that different groups at different sites obtain the same results with the same chips, and working out manufacturing and cost.
PA : We identified social barriers being an important obstacle to the transition, appearing to be much more the issue than the scientific ones, do you agree ?
Pr. D. Ingber : Yes. And toxicology is the worst. Toxicologists in companies say that the FDA is never going to buy this, no matter what they say. And then the FDA is basically always wanting to see more because they have the ultimate responsibility to ensure safety. It is a huge challenge that I don’t know exactly how we get through. I hope that the more OOC are used on the drug discovery side and they are proven to be accurate and valuable, the more people will have comfort. I remember the early days of the OOC, the toxicologists would not even be open to listening to the talks. It is changing, but there is still a lot of skepticism. I guess that is the reason they are good at what they do, they are very cautious. It is hard to change the way people have done things if they have done them for a long time.
PA : You mentioned that toxicology is among the most conservative fields. Do you think toxicology will nonetheless show some steps ahead soon ?
Pr. D. Ingber : I definitely think that everyone knows that the toxicology predictions of animals are suboptimal at best. The problem with animal testing is that they often don’t have the disease that the patient has. They are not old, they do not have multiple other diseases and they’re not on numerous different drugs. So there is a lot that you never see there. I do know there have been really nice results with kidney chips. The liver chips we already mentioned do also much better than animals. And those are two of the major toxicities. Companies are unfortunately going to be slow. They are still going to use animals. Although the executives of the companies see the value of these new approaches, nobody wants to be the one to take a risk. But I do think we are going to see less and less animals and more reliance on these alternative models. Toxicology is not only about identifying toxicity, it is also about understanding the molecular basis of that toxicity. OOC systems allow us to get into the mechanism of toxicity, and this is a huge value too. And for that value, these models will be used more. So I think we are definitely moving in the right direction. Everybody wants to see it happen.

PA : What are your main hopes and views for the near future and for the long-term ?
Pr. D. Ingber : I actually think that we are going to see OOC methods more widely used in the discovery and development pipeline before toxicology. I think there are many ways of accelerating drug discovery. There is no doubt that with the FDA Modernization Act, and with Emulate and many other companies distributing these methods and results internationally, that there will be more pharma and biotech companies using OOC and getting more comfortable with them. We are beginning to see that companies are finding use of Emulate’s liver chips can reduce costs significantly by reducing the need for studies with non-human primates. I hope that we will also see OOC integrated into clinical programs for precision medicine and clinical trials design.
I always say that the real game changer is that OOC can improve cost, shorten time, and enhance safety in drug development. Today, pharmaceutical groups spend hundreds of millions on the development of drugs, and tens of millions on big clinical trials that frequently fail. Then they search if there is a subpopulation for example, of genetically similar individuals — who responded better than others to the drug. If they find them, they then can do a targeted study with those patients, and if successful, they can get the drug approved for a narrow application. With OOC and patient-derived cells, I believe that we can flip that paradigm. We can make OOC for 50 or 100 patients to identify a given drug that works especially for them, and then use those same patients for a targeted clinical trial. In short, I see a future in which we leverage OOC to find the right drug that works most effectively for a defined subgroup of patients, based on very similar genetic subgroups, or on age, sex/gender, or on clinical manifestations. But the challenge we face with the replacement of animal studies is our risk averse culture and human nature — it really comes down to individuals taking risks. But people do change over time. That is how things change. And so again, once you see successes, and these become economic successes, then that will flip off the pendulum. Things will shift.
PA : We previously had the chance to interview Professor Thomas Hartung. He and his team started to combine brain organoids and Artificial Intelligence (AI). Did you get the chance to discuss with him about that and what perspective do you see in this new field ?
Pr. D. Ingber : I haven’t had a chance to talk with him about this new work. I’d love to see what he’s doing with it. We have been using AI for years and combining it with OOC. In fact, I am writing a proposal right now on using AI to communicate with our lymph node chip to get insight into immune responses. With our lymph node chip (4), we know that we can mimic vaccination responses, responses to infections, autoimmune responses, and so on. There are a lot of questions that we could ask and test whether predictions with AI are in fact correct. You need to have biological tests to be able to iterate and optimize, if you want to use AI. I think that AI will play a key role in the future of science and medicine, there is no doubt about it. At the Wyss Institute, we have used machine learning and various forms of AI for years. We have developed our own algorithms. We were able to use these algorithms with transcriptomic profiles from the literature, databases, and clinical studies, as well as OOC or other in vitro models, and to identify new clinical indications for existing drugs developed for other purposes, an approach known as drug repurposing. As an example, we published last year that our algorithm predicted that a subset of statin drugs, the most highly prescribed drugs in the world, used to lower cholesterol levels, would actually protect people against Covid-19 (5). By doing a retrospective study using electronic medical records of 4000 patients with Stanford University we confirmed that a subset of these statin drugs saved people’s lives and reduced death rates during the COVID-19 crisis. AI is going to be incredibly powerful, especially when combined with OOC, which can give you rich data. However, the problem with OOC, and the organoids such as the ones Hartung works with, is they are low throughput. So you need to be able to use other systems to generate huge amounts of data to train the AI — which we use from the literature and public databases — and then be able to counterpoint it with these high content in vitro models. AI is advancing so quickly that it is kind of amazing, but having the right data to train it and to develop ways to interface with these systems is really where challenges lie.
PA : To complete our interview, is there any other topic you would like to share with us and our readers ? Regarding the Wyss Institute, your next projects ?
Pr. D. Ingber : I do a lot of different things at the Wyss Institute. Organ chips are just a tiny bit of what I do. I also work on handheld diagnostics and therapeutics. Using the lung chips, we discovered a new RNA therapeutic that is sort of disease agnostic. It increases your interferon response — your host protective response — against SARS-CoV‑2 as well as SARS-CoV, MERS-CoV and influenza, infections of all types, but cancers as well. It has a dramatic protective effect in an animal COVID-19 model, and this all came out of our lung chip work.
And I think that is why I get excited about using these chips for discovery, not just toxicology. I find toxicology the least interesting thing. There are many ways to do toxicology experiments. Beyond this, we also are working in the area of brain health. Basically the reason there are no good drugs out there for most brain diseases is that very few drugs cross the blood brain barrier or BBB. So we are also working on shuttles to deliver drugs across the BBB, and we have come up with new shuttles that are incredibly exciting, in fact, we have already licensed multiple to pharma and biotech companies. So we do a lot of things in my group. The whole institute does unbelievable things.
Learn more about Pr Ingber
Image credit : Pr. D. Ingber, Wyss Institute Harvard
References
- Chou, D.B., Frismantas, V., Milton, Y. […] Ingber DE. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat Biomed Eng 4, 394 – 406 (2020). https://doi.org/10.1038/s41551-019‑0495‑z
- Si, L., Bai, H., Rodas, M. […] Ingber DE. A human-airway-on-a-chip for the rapid identification of candidate antiviral therapeutics and prophylactics. Nat Biomed Eng 5, 815 – 829 (2021). https://doi.org/10.1038/s41551-021 – 00718‑9
- Bai, H., Si L, Jiang A, Belgur C, […] Ingber DE. Mechanical control of innate immune responses against viral infection revealed in a human lung alveolus chip. Nat Commun. 2022 Apr 8;13(1):1928. https://doi.org/10.1038/s41467-022 – 29562‑4
- Goyal G, Prabhala P, Mahajan G, […] Ingber DE. Ectopic Lymphoid Follicle Formation and Human Seasonal Influenza Vaccination Responses Recapitulated in an Organ-on-a-Chip. Adv Sci (Weinh). 2022 May;9(14):e2103241. https://doi.org/10.1002%2Fadvs.202103241
- Sperry, M.M., Oskotsky TT, Marić I, […] Ingber DE. Target-agnostic drug prediction integrated with medical record analysis uncovers differential associations of statins with increased survival in COVID-19 patients. PLOS Computational Biology 19(5): e1011050 (2023) https://doi.org/10.1371/journal.pcbi.1011050


