
Endocrine disruptors
Let's take a closer look
Endocrine disruptors: Let’s take a closer look
Contribution from Dr. Jean-Pierre Cravedi, Toxicologist, former INRAE research director.
Key takeaways of this article
- Endocrine disruptors (EDs) impose or block natural hormones, affecting in particular the reproductive and thyroid systems and can lead to fertility and development disorders, hormone-dependent cancers, and chronic diseases (diabetes, neurodevelopmental disorders, etc.).
- ED are found in many everyday products (food, cosmetics, packaging) and can have an impact even at low concentrations, particularly during critical periods (e.g. perinatal).
- The effects of EDs may appear late and be transmitted to offspring and combined exposure to several EDs may amplify their effects (so-called cocktail effects).
- Current conventional toxicological methods are insufficient to fully assess ED.
- The challenges in assessing EDs call for the development of non-animal tests that are both faster to implement and more relevant to public health.
The hypothesis that the chemical substances to which we are exposed are the cause of hormonal dysfunctions and therefore lead to health disorders has been widely discussed in the scientific world over the past 30 years, and reported extensively in the media.
Originally, this hypothesis was based mainly on four types of work:
- Observations on wild species showing a causal link between exposure to one or more chemical substances and effects on reproduction or development, ultimately resulting in a very significant reduction in populations. This is the case, for example, of North Sea whelks decimated in the 1980s by tributyl-tin (the active substance in antifouling paints for boat hulls), or of male alligators in Florida’s Lake Apopka due to high exposure to organochlorine pesticides (Tohmé et al., 20101).
- Data collected on the consequences of in utero exposure to a synthetic estrogen, diethylstilbestrol (DES). This drug, prescribed to pregnant women between 1950 and 1977 to prevent miscarriage and pregnancy complications, caused infertility, vaginal cancer and numerous uterine abnormalities in young women whose mothers had received DES during pregnancy.2
- The effects on laboratory animals of substances capable of interfering with the endocrine system and impacting on the body functions regulated by these hormones. For example, numerous studies in rats have shown that certain phthalates, such as di-butyl phthalate, butyl-benzyl phthalate and diethylhexyl phthalate, are capable of reducing testosterone production (anti-androgenic effect), leading to fertility and development disorders.3
- Epidemiological studies attempting to establish a link between exposure to one or more chemical substances and hormone-dependent disorders, such as the impact of Dichlorodiphenyltrichloroethane (DDT, a highly stable and persistent organic pollutant) and its metabolites on development or lactation, or polychlorinated biphenyls (PCBs) on thyroid function.4
The combination of these initial data, acquired for the most part between 1975 and 1990, gave rise to the notion of endocrine disruption in 1991 at the Wingspread Conference (Wisconsin, USA). Several thousand scientific publications on the subject between 1990 and 2010, particularly on the health impact of endocrine disruptors (EDs), led the World Health Organization (WHO) to define them as “a substance or mixture of substances, which alters the functions of the endocrine system and thereby induces adverse effects in an intact organism, in its offspring or in (sub)-populations.” This definition was endorsed by the European Commission in 1999.
How do EDs work?
The endocrine system is based on a network of endocrine glands, capable of producing hormones, and hormone-targeted tissues, distributed throughout various organs. Hormones, most of which are transported by the bloodstream, enable the fine regulation of various body functions such as reproduction, growth, development and metabolism. Elements of the endocrine system include the hypothalamus, pituitary, thyroid and parathyroid glands, breasts, pancreas, adrenal glands, ovaries and testes (Figure 1).
This complex, multi-interactive and fragile system can be disrupted by substances from outside the body, leading to malfunctions in its essential functions. Historically, estrogens and androgens (female and male hormones respectively) were the first to be observed, raising awareness among scientists, public authorities and, more broadly, the general public of the need to gain a better understanding of endocrine disruption. Over the last ten years or so, other hormones/targets have been explored, considerably broadening the ED issue. In particular, numerous publications have reported on chemical agents likely to affect thyroid function, metabolic regulation or brain development by interfering with the hormones that regulate these balances.
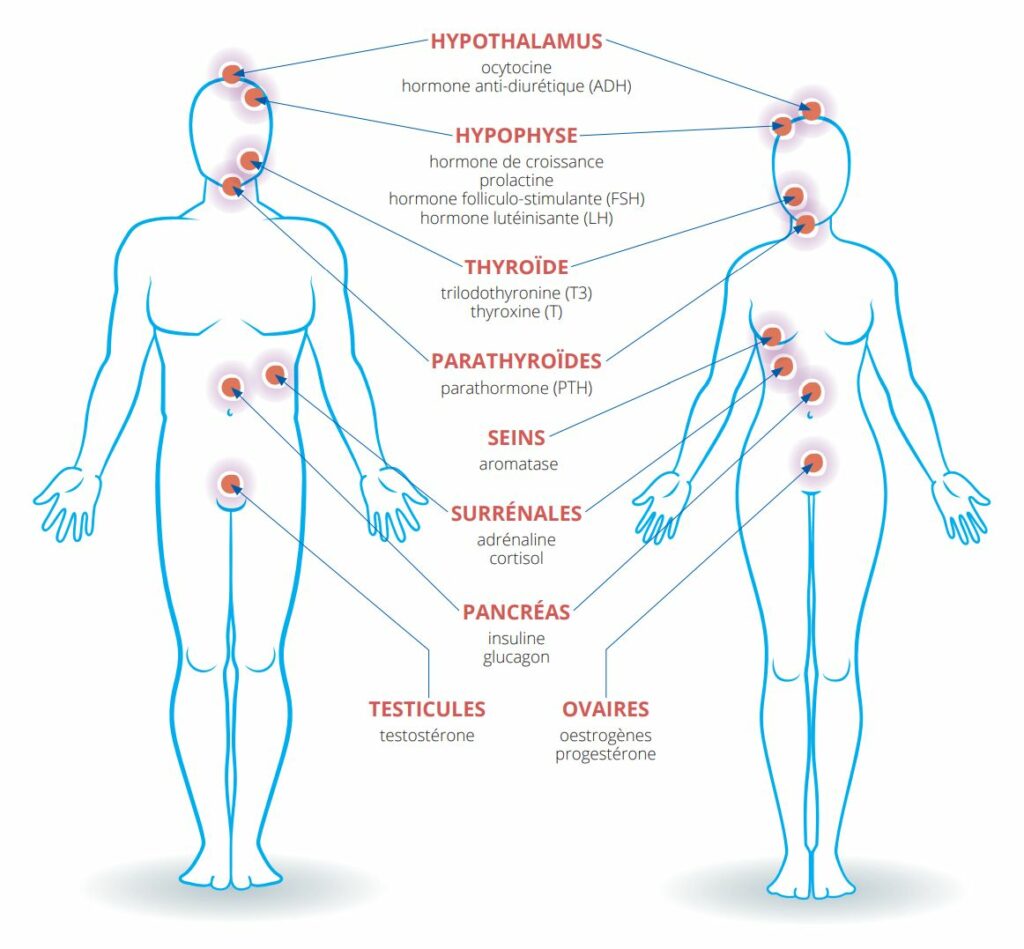
Figure 1: The male and female endocrine systems and the main hormones likely to be affected by endocrine disruptors. Illustration inspired by the Pepper Platform
The complexity of the ED issue lies not only in the diversity of targets involved, but also in the multiplicity of these substances’ modes of action. Indeed, the mechanism by which hormones elicit a physiological response is essentially based on the binding of the hormone to its cellular receptor(s). These receptors are sometimes compared to a lock in which the hormone comes to lodge, acting as a key and provoking an action.
It’s therefore easy to imagine that a ED can interfere with the hormonal system by substituting itself for the real hormone and imitating its action. This is referred to as the substance’s mimetic or agonist effect. It is also possible, to use the image evoked earlier, for the substance to block the “lock”, preventing the hormone from attaching to its receptor and rendering its action impossible. In this case, the substance has an antagonistic effect.
In addition to these types of mechanisms via hormone receptors, EDs can also alter hormone synthesis, transport or degradation. All these modes of action are illustrated in Figure 2.
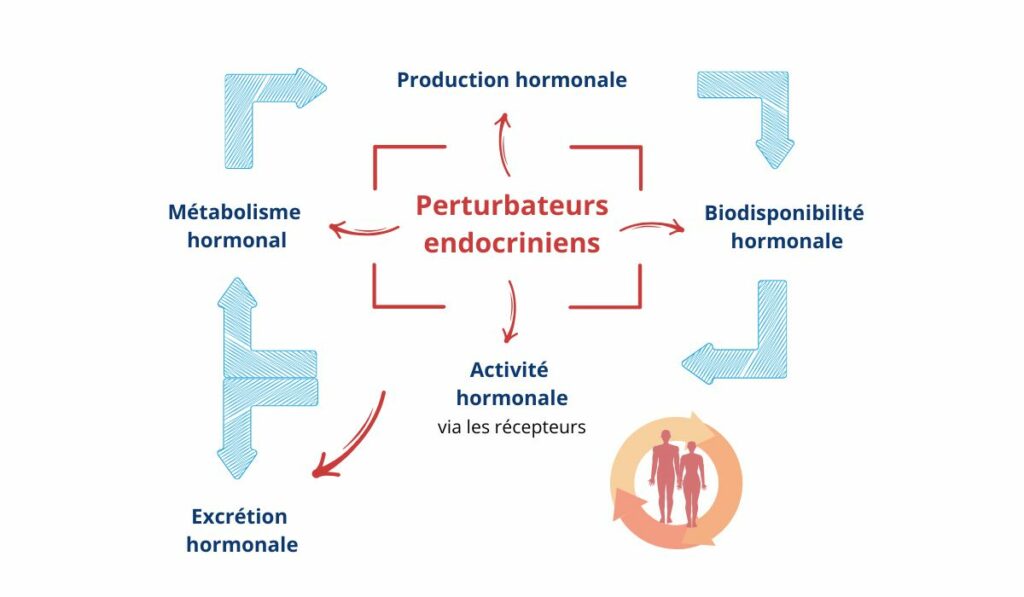
Figure 2 : Main modes of action of endocrine disruptors
Several factors add to the complexity of these mechanisms and make interpretation of the data tricky. Firstly, the fact that the same hormone can originate from different organs and act on different target tissues that do not necessarily respond to the same circulating doses represents a first difficulty. For example, estradiol, an estrogen mainly synthesized in the ovaries and necessary for the maintenance of fertility and secondary sexual characteristics in women, is also produced in the placenta and, to a much lesser extent, in the testes. In addition, there are not one but several estrogen receptors, which do not lead to the same effects, and lastly, these receptors are located in different tissues which are sometimes not directly linked to the reproductive organs, making it difficult to understand the mechanisms of action of these receptors and therefore the substances likely to affect them.
A case in point is the presence of estrogen receptors in the intestine, which affect its permeability and consequently its function as a nutrient absorber and barrier to biological and chemical aggressors. Also on the subject of estrogen-mimetic compounds, bisphenol A (used in the composition of certain plastics and resins) is capable of binding to estrogen receptors and thus triggering the same type of response as female hormones, but it can also block the androgen receptor, i.e. the one that responds to male hormones such as testosterone.
These multiple modes of action, which sometimes vary according to dose, are far from exceptional. PCBs (polychlorinated biphenyls, formerly mainly used as electrical insulators or heat-transfer fluids, but still present in the environment and the food chain) acting as such or after being transformed in the body, are capable not only of acting on various hormone receptors, but also on the synthesis, transport or degradation of hormones such as sex, thyroid, adrenal or metabolic hormones.5
What are EDs and where can they be found?
It is difficult to draw up an inventory of EDs. They are present in many everyday objects and products (detergents, household products, biocides, cosmetics, food products, packaging, medical devices, toys, etc.), and new ones are being discovered all the time.
Among the product families most concerned are :
- Pesticides: insecticides (e.g. DDT, chlordecone, endosulfan, pyrethroids), herbicides (e.g. atrazine, linuron, 2,4 D), fungicides (e.g. benomyl, maneb, zineb, hexachlorobenzene, epiconazole).
- Heavy metals: cadmium, lead, mercury
- Products used in cosmetics: parabens, benzophenones
- Industrial products: alkylphenols, bisphenols, phthalates, perchlorates, PFAS
- Contaminants: dioxins, PCBs, polycyclic aromatic hydrocarbons
- Natural substances: phytoestrogens (e.g. genistein, coumestrol), mycotoxins (e.g. zearalenone)
Let’s also remember that many drugs such as contraceptives or certain anti-cancer drugs and especially those used to treat hormone-dependent cancers are necessarily EDs since their mode of action intervenes on the hormonal system.
What do the regulations say?
Although consideration of EDs appeared as early as 2006 in the regulation concerning the Registration, Evaluation and Authorization of Chemicals in Europe (REACH), and was mentioned in the regulations on plant protection products in 2009 and on biocidal products in 2012, it wasn’t until 2017 that the scientific criteria for determining EDs were defined. These criteria require (1) that the substance has an adverse effect (modification of the morphology, physiology, growth, development, reproduction or lifespan of an organism) that leads to an alteration in health; (2) that it has an endocrine mode of action, i.e. that it alters the function(s) of the endocrine system; (3) that the adverse effect is a consequence of the endocrine mode of action.
However, strictly speaking, there is no single official list of all substances classified as ED. Within the EU, several inventories coexist depending on the regulations covering these products. For example, for substances falling within the scope of the REACH regulation, the list regularly updated by the European Chemicals Agency (ECHA) currently includes a dozen substances or groups of substances classified as ED on the basis of their effects on human health.6 Nearly 150 substances have been or are being evaluated by this agency, and more than twenty of them have been classified as ED on the basis of their environmental impact. Other lists concerning plant protection products and biocides are being drawn up by the competent authorities.
In 2022, EDs will also make their appearance in the CLP regulation on the classification, labeling and packaging of chemical substances.
The national authorities of Belgium, Denmark, France, the Netherlands, Sweden and Spain have taken the initiative of grouping together substances classified as ED on a single site.7 This information is detailed in two separate lists (List I and II), based on the EU regulations for REACH (Registration, Evaluation, Authorization and Restriction of Chemicals), for biocidal products, for plant protection products and for cosmetic products. A third list (List III) details substances which may have ED properties, and which have been proposed by an individual national authority. As such, these inclusions are not necessarily supported by other EU member states.
It should also be noted that in France, EDs have been the subject of two national strategies (SNPE1 and SNPE2), aimed in particular at reducing exposure of the environment and the population to these substances. Since 2022, France’s anti-waste law for a circular economy (AGEC) has required consumers to be informed about the possible presence of Es in products placed on the market. This information must be made available on the Internet, in full public access and free of charge.
What are the health effects?
A large proportion of studies on EDs initially highlighted fertility and reproductive disorders (reduced sperm quality, increased frequency of abnormalities in the development of reproductive organs or reproductive function, lowering of the age of puberty), as well as developmental effects, or an impact on hormone-dependent cancers, such as breast or prostate cancers8. Several studies have also established a link between endocrine disruption and endometriosis.9 More recent scientific data suggest that they can alter the functioning of many other organs and body functions (altered immune system, thyroid disorders, liver disease, etc…). Neurodevelopmental disorders such as autism, lower IQ or metabolic disorders such as diabetes or obesity are also suspected to be associated with exposure to ED10.Damage to bone, skin or eye physiology has also been reported11.
Nevertheless, the causal link between the action of a ED and its adverse effects in the general population is difficult to establish due to the fact that pathologies linked to endocrine disruption, which are generally chronic, are for the most part multifactorial.5 Santé publique France considers that 21 health effects of EDs are to be monitored as a priority.12 Among them are six reproductive health effects (cryptorchidism, hypospadias, precocious puberty, testicular cancer, impaired sperm quality and endometriosis), to which infertility and reduced fertility are added. Also included are metabolic effects (overweight and obesity, cardiovascular disease, type 2 diabetes and metabolic syndrome), neurodevelopmental disorders in children (behavioral, intellectual and attention deficit disorders), cancers (breast cancer, prostate cancer, lymphoma and leukemia in children), and asthma.
The singularity of EDs in risk assessment
EDs have a number of special features that justify their being given a special place in risk assessment.
Firstly, although they are not the only ones in this case, their effects can occur at very low doses, which is not surprising since they often mimic hormones, which have the property of acting at minute concentrations.
Then, more than dose or duration, it’s the exposure period that plays a decisive role. The most sensitive “window” of exposure for a majority of EPs is the perinatal period, i.e. the period covering foetal life and early childhood. This period is the source of most of the effects attributed to certain phthalates and bisphenols, for example. Puberty is also a period of vulnerability to EDs.
Furthermore, in line with Barker’s hypothesis that what happens during prenatal development has a direct impact on health and the development of chronic diseases in adulthood, the effects of EPs can appear long after exposure. The example of DES cited earlier is a case in point.
Beyond these delayed impacts, experimental work on rodents with EDs such as vinchlozoline, a now-banned fungicide, bisphenol A or even certain phthalates shows that the effects observed in the exposed individual can be transmitted over several generations (multigenerational or transgenerational effects). The mechanisms involved are probably epigenetic, i.e. involve changes in gene expression without changing the DNA sequence.
The fifth peculiarity concerns non-monotonic dose-response curves, which have been demonstrated in several experimental studies involving EDs. Toxicologists are accustomed to saying that the dose makes the poison, in other words that the higher the dose, the greater the effect. When these results are plotted on a graph, we speak of a monotonic curve, which makes it easy to derive a no-effect dose and thus propose a threshold below which there is no risk. On the other hand, for some EDs, it can happen that low doses have greater, or even opposite, effects to those of higher doses, resulting in U‑shaped or inverted-U-shaped dose-response curves and calling into question the usual methods of risk assessment. However, an in-depth analysis of the scientific literature conducted by the European Food Safety Authority (EFSA) and several national risk assessment agencies, including Anses for France, suggests that this phenomenon is more the exception than the rule.13
Finally, the “cocktails” of EDs can give rise to synergistic or potentiating effects, i.e. the effect of the mixture can be greater than that of each of its components taken individually. While this effect is not specific to EDs, it has been particularly highlighted in this case because of the multiple targets on which they can act. Exploration of the mechanisms behind these synergies has demonstrated that interactions are possible within the very receptors to which EDs bind.14
What do we know about human exposure to EDs?
EDs are widely distributed in our environment. In the general population, exposure can occur in many different ways.
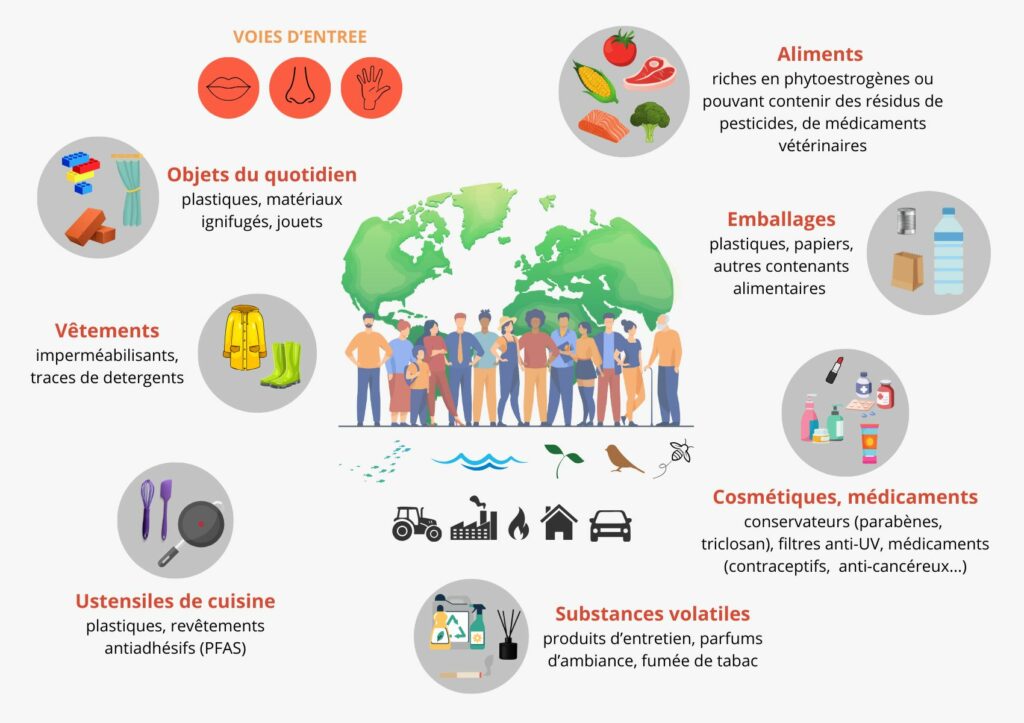
Figure 3 : Endocrine disruptors: multiple exposures.
Population impregnation studies show that EDs are present in all children and adults in whom measurements of substances recognized as EDs have been measured.15
What toxicological tests are available?
There is a chain comprising a succession of tests standardized by the OECD, classified into three levels: tests in silico, tests in vitro and tests in vivo.16 Tests in silico examine the structure of the product and model potential binding to a hormone receptor. In vitro tests reveal the mechanism of action: binding to hormone receptors, interaction with hormone transport proteins, or effect on hormone synthesis. Tests in vivo, often carried out on rodents, involve investigating estrogenic, androgenic, or antagonistic effects on organs, organisms and over several generations. There are also tests on fish and amphibians.
These tests, developed for regulatory applications (e.g. REACH regulations), do however have their limitations, particularly with regard to the limited number of targets examined. While the synthesis process of steroid hormones is well taken into account, the hormonal regulations considered are essentially those regulated by estrogens, androgens and thyroid hormones. Moreover, they remain incomplete with regard to several aspects of the ED issue (effect of low doses, cocktail effects, exposure window).
In 2018, ECHA and EFSA, with the help of the European Commission’s Joint Research Center (JRC), published a guidance document on the identification of EDs under the regulations on biocides and plant protection products.17 This guide, which could be applied to substances other than those for which it was designed, takes into account in its approach not only regulatory tests, but also all available data, in a “weight of evidence” approach (weight of evidence) concerning both modes of action and toxic effects.
More recently, the US Environmental Protection Agency (US-EPA) published a report demonstrating the value of New Methodological Approaches (NMAs), i.e., placing a large emphasis on in silico, in vitro and mathematical modeling to apprehend the risk associated with EDs.18
In addition, there are many other non-standardized tests, the use of which raises the question of the reliability of results and their acceptance at international level. Research and development efforts therefore need to be stepped up to provide a battery of tests that better cover the many potential targets of EDs, and to ensure the validity of the methods used, so that the data produced can lead to better risk assessment by health agencies.
CONCLUSION
Despite thousands of publications on EDs, the risk they pose to public health remains difficult to assess. An incomplete battery of biological tests, tricky animal-human extrapolation, sometimes discordant data in humans, make it difficult to demonstrate a causal link between exposure data and possible hormonal dysfunction. An increase in health disorders due to EDs is all the more difficult to demonstrate as these are likely to appear several years or decades after pre- or post-natal exposure, to these substances. In the face of these uncertainties, there is nonetheless significant scientific evidence, indisputable regulatory and risk management advances, and emerging health measures that should be welcomed and supported.
A supplement to this article will be given in 2025 and also published online and in the journal to further explore non-animal testing methods as applied to EDs, addressing their challenges and how these methods might address and/or contribute to resolving some of the uncertainties in current knowledge and assessments.
References
[1] Thomé M, Cravedi JP et Laudé V. Des polluants hormonaux, Pour la Science N°396 — octobre 2010.
[2] Fillion E, Torny D, un précédent manqué : le Distilbène® et les perturbateurs endocriniens. Contribution à une sociologie de l’ignorance, Sciences Sociales et Santé, Vol. 34, n° 3, septembre 2016.
[3] Silano V, Barat Baviera JM, Bolognesi C, et al. Update of the risk assessment of DBP, BBP, DEHP, DINP and DIDP for use in food contact materials. EFSA J. 2019 Dec 11;17(12):e05838.
[4] Cravedi JP, Narbonne JF. Données récentes sur l’évaluation des dangers liés à la présence de PCB dans l’alimentation, cité dans l’avis de l’AFSSA du 8 avril 2003. Décembre 2002.
[5] Les perturbateurs endocriniens, Actualité et dossier en santé publique n° 115, Haut Conseil de la Santé Publique (HCSP).
[6] Endocrine disruptor assessment list, ECHA (Last updated 08 novembre 2024).
[7] Endocrine disruptor list, The ED Lists.
[8] Darbre PD. The history of endocrine-disrupting chemicals. Current Opinion in Endocrine and Metabolic Research 2019, 7. pp. 26 – 33. ISSN 2451 – 9650.
[9] Dutta S, Banu SK, Arosh JA. Endocrine disruptors and endometriosis. Reprod Toxicol. 2023 Jan;115:56 – 73.
[10] Gore, AC., La Merrill, MA, Patisaul, HB, et al. Endocrine Disrupting Chemicals: Threats to Human Health. The Endocrine Society and IPEN. February 2024. ISBN # 978 – 1‑955400 – 23‑7.
[11] Shulhai AM, Palanza P, Street, ME. Current Evidence on the Effects of Endocrine-Disrupting Chemicals (EDCs) on Bone Growth and Health. Expo Health 16, 1001 – 1025 (2024).
[12] Vers un élargissement de la surveillance des effets sanitaires des perturbateurs endocriniens, Santé Publique France — décembre 2023.
[13] Beausoleil et al. Review of non-monotonic dose-responses of substances for human risk assessment. EFSA supporting publication 2016:EN-1027. 290pp
[14] « L’effet cocktail » des perturbateurs endocriniens mieux compris, INSERM Presse — janvier 2021.
[15] Perturbateurs endocriniens, Santé Gouv — Dernière mise à jour août 2024.
[16] Revised Guidance Document 150 on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption, OECD — September 2018.
[17] Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009, EFSA — June 2018.
[18] EPA Rebuilds Endocrine Disruptor Screening Program by Soliciting Public Comment on New Approach Methodologies to Screen for Endocrine Effects, US EPA — January 2023.
About the author
Dr Jean-Pierre Cravedi
 Toxicologist, Chairman of Aprifel’s Scientific Advisory Board, former ANSES and EFSA expert.
Toxicologist, Chairman of Aprifel’s Scientific Advisory Board, former ANSES and EFSA expert.
A former INRAE research director, he headed the Xenobiotics UMR in Toulouse, before becoming deputy head of the Human Food Department from 2014 to 2019. His work has led him to study the fate and effects of several contaminants present in the environment or in food, including endocrine disruptors.
Since 2025, Dr Jean-Pierre Cravedi has also chaired the strategic committee of the Descroix-Vernier EthicScience Prize.
- Thomé M, Cravedi JP et Laudé V. Des polluants hormonaux, Pour la Science N°396 — octobre 2010.
- Fillion E, Torny D, un précédent manqué : le Distilbène® et les perturbateurs endocriniens. Contribution à une sociologie de l’ignorance, Sciences Sociales et Santé, Vol. 34, n° 3, septembre 2016
- EFSA J. 2019 Dec 11;17(12):e05838.
- Cravedi JP, Narbonne JF. Données récentes sur l’évaluation des dangers liés à la présence de PCB dans l’alimentation, cité dans l’avis de l’AFSSA du 8 avril 2003. Décembre 2002. Disponible sur www.afssa.fr
- https://www.hcsp.fr/explore.cgi/adsp?clef=1176
- https://echa.europa.eu/fr/ed-assessment
- https://edlists.org/
- Darbre PD. The history of endocrine-disrupting chemicals. Current Opinion in Endocrine and Metabolic Research 2019, 7:26 – 33
- Dutta S, Banu SK, Arosh JA. Endocrine disruptors and endometriosis. Reprod Toxicol. 2023 Jan;115:56 – 73.
- Gore, AC., La Merrill, MA, Patisaul, HB, et al. Endocrine Disrupting Chemicals: Threats to Human Health. The Endocrine Society and IPEN. February 2024. ISBN # 978 – 1‑955400 – 23‑7
- Shulhai AM, Palanza P, Street, ME. Current Evidence on the Effects of Endocrine-Disrupting Chemicals (EDCs) on Bone Growth and Health. Expo Health 16, 1001 – 1025 (2024). https://doi.org/10.1007/s12403-023 – 00607‑3
- https://www.santepubliquefrance.fr/les-actualites/2023/vers-un-elargissement-de-la-surveillance-des-effets-sanitaires-des-perturbateurs-endocriniens
- Beausoleil et al, 2016. Review of non-monotonic dose-responses of substances for human risk assessment. EFSA supporting publication 2016:EN-1027. 290pp
- https://presse.inserm.fr/leffet-cocktail-des-perturbateurs-endocriniens-mieux-compris/41920/
- https://sante.gouv.fr/sante-et-environnement/risques-microbiologiques-physiques-et-chimiques/article/perturbateurs-endocriniens
- https://www.oecd.org/en/publications/guidance-document-on-standardised-test-guidelines-for-evaluating-chemicals-for-endocrine-disruption-2nd-edition_9789264304741-en.html
- https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2018.5311
- https://www.epa.gov/pesticides/epa-rebuilds-endocrine-disruptor-screening-program-soliciting-public-comment-new


